Beneficial role of the GPR30 agonist G-1 in an animal model of multiple sclerosis
- PMID: 19664827
- PMCID: PMC2873862
- DOI: 10.1016/j.jneuroim.2009.06.023
Beneficial role of the GPR30 agonist G-1 in an animal model of multiple sclerosis
Abstract
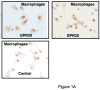
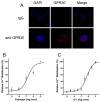
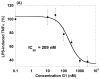
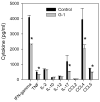
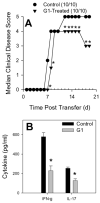
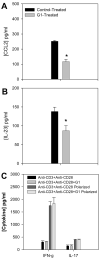
The beneficial effects of estrogens in multiple sclerosis are thought to be mediated exclusively by the classical nuclear estrogen receptors ERalpha and ERbeta. However, recently many reports revealed that estrogens are able to mediate rapid signals through a G protein-coupled receptor (GPCR), known as GPR30. In the present study, we set out to explore whether effects mediated through this receptor were anti-inflammatory and could account for some of the beneficial effects of estrogen. We demonstrate that GPR30 is expressed in both human and mouse immune cells. Furthermore a GPR30-selective agonist, G-1, previously described by us, inhibits the production of lipopolysaccharide (LPS)-induced cytokines such as TNF-alpha and IL-6 in a dose-dependent manner in human primary macrophages and in a murine macrophage cell line. These effects are likely mediated solely through the estrogen-specific receptor GPR30 since the agonist G-1 displayed an IC(50) far greater than 10 microM on the classical nuclear estrogen receptors as well as a panel of 25 other GPCRs. Finally, we show that the agonist G-1 is able to reduce the severity of disease in both active and passive EAE models of multiple sclerosis in SJL mice and that this effect is concomitant with a G-1-mediated decrease in proinflammatory cytokines, including IFN-gamma and IL-17, in immune cells harvested from these mice. The effect of G-1 appears indirect, as the GPR30 agonist did not directly influence IFN-gamma or IL-17 production by purified T cells. These data indicate that G-1 may represent a novel therapeutic agent for the treatment of chronic autoimmune, inflammatory diseases.
Figures







References
-
- Abramsky O. Pregnancy and multiple sclerosis. Ann Neurol. 1994;36(Suppl):S38–41. - PubMed
-
- Arnason BG, Richman DP. Effects of estrogen, progestin and combined estrogen-progestin oral contraceptive preparations on experimental allergic encephalomyelitis. Trans Am Neurol Assoc. 1969;94:54–58. - PubMed
-
- Bologa CG, Revankar CM, Young SM, Edwards BS, Arterburn JB, Kiselyov AS, Parker MA, Tkachenko SE, Savchuck NP, Sklar LA, Oprea TI, Prossnitz ER. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol. 2006;2:207–212. - PubMed
-
- Carmeci C, Thompson DA, Ring HZ, Francke U, Weigel RJ. Identification of a gene (GPR30) with homology to the G-protein-coupled receptor superfamily associated with estrogen receptor expression in breast cancer. Genomics. 1997;45:607–617. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

