MAP kinase dependent cyclinE/cdk2 activity promotes DNA replication in early sea urchin embryos
- PMID: 19665013
- PMCID: PMC2789238
- DOI: 10.1016/j.ydbio.2009.07.043
MAP kinase dependent cyclinE/cdk2 activity promotes DNA replication in early sea urchin embryos
Abstract
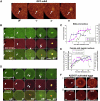
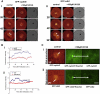
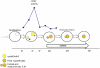
Sea urchins provide an excellent model for studying cell cycle control mechanisms governing DNA replication in vivo. Fertilization and cell cycle progression are tightly coordinated by Ca(2+) signals, but the mechanisms underlying the onset of DNA replication after fertilization remain less clear. In this study we demonstrate that calcium-dependent activation of ERK1 promotes accumulation of cyclinE/cdk2 into the male and female pronucleus and entry into first S-phase. We show that cdk2 activity rises quickly after fertilization to a maximum at 4 min, corresponding in timing to the early ERK1 activity peak. Abolishing MAP kinase activity after fertilization with MEK inhibitor, U0126, substantially reduces the early peak of cdk2 activity and prevents cyclinE and cdk2 accumulation in both sperm pronucleus and zygote nucleus in vivo. Both p27(kip1) and roscovitine, cdk2 inhibitors, prevented DNA replication suggesting cdk2 involvement in this process in sea urchin. Inhibition of cdk2 activity using p27(kip1) had no effect on the phosphorylation of MBP by ERK, but completely abolished phosphorylation of retinoblastoma protein, a cdk2 substrate, indicating that cdk2 activity is downstream of ERK1 activation. This pattern of regulation of DNA synthesis conforms to the pattern observed in mammalian somatic cells.
Figures




Similar articles
-
Cdk2 activity is dispensable for the onset of DNA replication during the first mitotic cycles of the sea urchin early embryo.Dev Biol. 1998 Aug 15;200(2):182-97. doi: 10.1006/dbio.1998.8961. Dev Biol. 1998. PMID: 9705226
-
Cyclin E/Cdk2 is required for sperm maturation, but not DNA replication, in early sea urchin embryos.Genesis. 2007 May;45(5):282-91. doi: 10.1002/dvg.20291. Genesis. 2007. PMID: 17458867
-
ERK1 activation is required for S-phase onset and cell cycle progression after fertilization in sea urchin embryos.Development. 2005 Feb;132(3):579-89. doi: 10.1242/dev.01607. Epub 2005 Jan 5. Development. 2005. PMID: 15634691
-
Aberrant cell cycle regulation in cervical carcinoma.Yonsei Med J. 2005 Oct 31;46(5):597-613. doi: 10.3349/ymj.2005.46.5.597. Yonsei Med J. 2005. PMID: 16259056 Free PMC article. Review.
-
Cdk2 as a master of S phase entry: fact or fake?Cell Cycle. 2004 Jan;3(1):35-7. Cell Cycle. 2004. PMID: 14657662 Review.
Cited by
-
ANGPTL3 is a novel biomarker as it activates ERK/MAPK pathway in oral cancer.Cancer Med. 2015 May;4(5):759-69. doi: 10.1002/cam4.418. Epub 2015 Jan 30. Cancer Med. 2015. PMID: 25644496 Free PMC article.
-
ERK1/2 promotes cigarette smoke-induced rat pulmonary artery smooth muscle cells proliferation and pulmonary vascular remodeling via up-regulating cycline1 expression.J Huazhong Univ Sci Technolog Med Sci. 2013 Jun;33(3):315-322. doi: 10.1007/s11596-013-1117-8. Epub 2013 Jun 17. J Huazhong Univ Sci Technolog Med Sci. 2013. PMID: 23771653
-
MAPK phosphorylation of connexin 43 promotes binding of cyclin E and smooth muscle cell proliferation.Circ Res. 2012 Jul 6;111(2):201-11. doi: 10.1161/CIRCRESAHA.112.272302. Epub 2012 May 31. Circ Res. 2012. PMID: 22652908 Free PMC article.
-
Annexin A10 in human oral cancer: biomarker for tumoral growth via G1/S transition by targeting MAPK signaling pathways.PLoS One. 2012;7(9):e45510. doi: 10.1371/journal.pone.0045510. Epub 2012 Sep 17. PLoS One. 2012. PMID: 23029062 Free PMC article.
-
Integrins on eggs: focal adhesion kinase is activated at fertilization, forms a complex with integrins, and is necessary for cortex formation and cell cycle initiation.Mol Biol Cell. 2013 Nov;24(21):3472-81. doi: 10.1091/mbc.E13-03-0148. Epub 2013 Aug 28. Mol Biol Cell. 2013. PMID: 23985318 Free PMC article.
References
-
- Cameron L., Poccia D. In vitro development of the sea urchin male pronucleus. Dev. Biol. 1994;162:568–578. - PubMed
-
- Carroll D.J., Albay D.T., Hoang K.M., O'Neil F.J., Kumano M., Foltz K.R. The relationship between calcium, MAP kinase, and DNA synthesis in sea urchin egg at fertilization. Dev. Biol. 2000;217:179–191. - PubMed
-
- Chambard J.C., Lefloch R., Pouyssegur J., Lenormand P. ERK implication in cell cycle regulation. Bioch. Bioph. Acta November. 2006;17:1–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

