Ras pathway activation in hepatocellular carcinoma and anti-tumoral effect of combined sorafenib and rapamycin in vivo
- PMID: 19665249
- PMCID: PMC2970800
- DOI: 10.1016/j.jhep.2009.03.028
Ras pathway activation in hepatocellular carcinoma and anti-tumoral effect of combined sorafenib and rapamycin in vivo
Abstract
Background/aims: The success of sorafenib in the treatment of advanced hepatocellular carcinoma (HCC) has focused interest on the role of Ras signaling in this malignancy. We investigated the molecular alterations of the Ras pathway in HCC and the antineoplastic effects of sorafenib in combination with rapamycin, an inhibitor of mTOR pathway, in experimental models.
Methods: Gene expression (qRT-PCR, oligonucleotide microarray), DNA copy number changes (SNP-array), methylation of tumor suppressor genes (methylation-specific PCR) and protein activation (immunohistochemistry) were analysed in 351 samples. Anti-tumoral effects of combined therapy targeting the Ras and mTOR pathways were evaluated in cell lines and HCC xenografts.
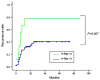
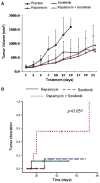
Results: Different mechanisms accounted for Ras pathway activation in HCC. H-ras was up-regulated during different steps of hepatocarcinogenesis. B-raf was overexpressed in advanced tumors and its expression was associated with genomic amplification. Partial methylation of RASSF1A and NORE1A was detected in 89% and 44% of tumors respectively, and complete methylation was found in 11 and 4% of HCCs. Activation of the pathway (pERK immunostaining) was identified in 10.3% of HCC. Blockade of Ras and mTOR pathways with sorafenib and rapamycin reduced cell proliferation and induced apoptosis in cell lines. In vivo, the combination of both compounds enhanced tumor necrosis and ulceration when compared with sorafenib alone.
Conclusions: Ras activation results from several molecular alterations, such as methylation of tumor suppressors and amplification of oncogenes (B-raf). Sorafenib blocks signaling and synergizes with rapamycin in vivo, preventing tumor progression. These data provide the rationale for testing this combination in clinical studies.
Figures




Comment in
-
Show me your signaling--and I'll tell you who you are.J Hepatol. 2009 Oct;51(4):638-9. doi: 10.1016/j.jhep.2009.05.011. Epub 2009 May 27. J Hepatol. 2009. PMID: 19592126 No abstract available.
References
-
- Sangiovanni A, Del Ninno E, Fasani P, De Fazio C, Ronchi G, Romeo R, et al. Increased survival of cirrhotic patients with a hepatocellular carcinoma detected during surveillance. Gastroenterology. 2004;126:1005–1014. - PubMed
-
- El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. - PubMed
-
- Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. - PubMed
-
- Villanueva A, Newell P, Chiang DY, Friedman SL, Llovet JM. Genomics and signaling pathways in hepatocellular carcinoma. Semin Liver Dis. 2007;27:55–76. - PubMed
-
- Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer. 2006;6:674–687. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

