Structural and functional changes in tau mutant mice neurons are not linked to the presence of NFTs
- PMID: 19665462
- PMCID: PMC2864360
- DOI: 10.1016/j.expneurol.2009.07.029
Structural and functional changes in tau mutant mice neurons are not linked to the presence of NFTs
Abstract
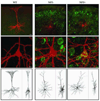
In the rTg4510 mouse model, expression of the mutant human tau variant P301L leads to development of neurofibrillary tangles (NFTs), neuronal death, and memory impairment, reminiscent of the pathology observed in human tauopathies. In the present study, we examined the effects of mutant tau expression on the electrophysiology and morphology of individual neurons using whole-cell patch-clamp recordings and biocytin filling of pyramidal cells in cortical slices prepared from rTg4510 (TG) and wild-type (WT) littermate mice. Among the TG cells, 42% contained a clear Thioflavin-S positive inclusion in the soma and were categorized as NFT positive (NFT+), while 58% had no discernable inclusion and were categorized as NFT negative (NFT-). The resting membrane potential (V(r)) was significantly depolarized (+8 mV) in TG cells, and as a consequence, evoked repetitive action potential (AP) firing rates were also significantly increased. Further, single APs were significantly shorter in duration in TG cells and the depolarizing voltage deflection or "sag" evoked by hyperpolarization was significantly greater in amplitude. In addition to these functional electrophysiological changes, TG cells exhibited significant morphological alterations, including loss or significant atrophy of the apical tuft, reduced dendritic complexity and length, and reduced spine density. Importantly, NFT- and NFT+ TG cells were indistinguishable with regard to both morphological and electrophysiological properties. Our observations show that expression of mutated tau results in significant structural and functional changes in neurons, but that these changes occur independent of mature NFT formation.
Copyright (c) 2009 Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Electrophysiological changes precede morphological changes to frontal cortical pyramidal neurons in the rTg4510 mouse model of progressive tauopathy.Acta Neuropathol. 2012 Dec;124(6):777-95. doi: 10.1007/s00401-012-1038-9. Epub 2012 Sep 14. Acta Neuropathol. 2012. PMID: 22976049 Free PMC article.
-
Changes in dendritic complexity and spine morphology in transgenic mice expressing human wild-type tau.Brain Struct Funct. 2010 Mar;214(2-3):161-79. doi: 10.1007/s00429-010-0245-1. Epub 2010 Mar 7. Brain Struct Funct. 2010. PMID: 20213269 Free PMC article.
-
Neurofibrillary tangle formation by introducing wild-type human tau into APP transgenic mice.Acta Neuropathol. 2014 May;127(5):685-98. doi: 10.1007/s00401-014-1259-1. Epub 2014 Feb 15. Acta Neuropathol. 2014. PMID: 24531886
-
[Analysis of mouse model exhibiting neurofibrillary changes].Rinsho Shinkeigaku. 2001 Dec;41(12):1111-2. Rinsho Shinkeigaku. 2001. PMID: 12235811 Review. Japanese.
-
Regulatable transgenic mouse models of Alzheimer disease: onset, reversibility and spreading of Tau pathology.FEBS J. 2013 Sep;280(18):4371-81. doi: 10.1111/febs.12250. Epub 2013 Apr 22. FEBS J. 2013. PMID: 23517246 Review.
Cited by
-
A Peephole into the Brain: Neuropathological Features of Alzheimer's Disease Revealed by in vivo Two-Photon Imaging.Front Psychiatry. 2012 Apr 2;3:26. doi: 10.3389/fpsyt.2012.00026. eCollection 2012. Front Psychiatry. 2012. PMID: 22485096 Free PMC article.
-
Analyzing dendritic spine pathology in Alzheimer's disease: problems and opportunities.Acta Neuropathol. 2015 Jul;130(1):1-19. doi: 10.1007/s00401-015-1449-5. Epub 2015 Jun 11. Acta Neuropathol. 2015. PMID: 26063233 Free PMC article. Review.
-
Curcumin suppresses soluble tau dimers and corrects molecular chaperone, synaptic, and behavioral deficits in aged human tau transgenic mice.J Biol Chem. 2013 Feb 8;288(6):4056-65. doi: 10.1074/jbc.M112.393751. Epub 2012 Dec 21. J Biol Chem. 2013. PMID: 23264626 Free PMC article.
-
Differential expression of tau species and the association with cognitive decline and synaptic loss in Alzheimer's disease.Alzheimers Dement. 2022 Sep;18(9):1602-1615. doi: 10.1002/alz.12518. Epub 2021 Dec 7. Alzheimers Dement. 2022. PMID: 34873815 Free PMC article.
-
Selective Disruption of Inhibitory Synapses Leading to Neuronal Hyperexcitability at an Early Stage of Tau Pathogenesis in a Mouse Model.J Neurosci. 2020 Apr 22;40(17):3491-3501. doi: 10.1523/JNEUROSCI.2880-19.2020. Epub 2020 Apr 7. J Neurosci. 2020. PMID: 32265258 Free PMC article.
References
-
- Andorfer C, Kress Y, Espinoza M, de Silva R, Tucker KL, Barde YA, Duff K, Davies P. Hyperphosphorylation and aggregation of tau in mice expressing normal human tau isoforms. J Neurochem. 2003;86:582–590. - PubMed
-
- Ballatore C, Lee VM, Trojanowski JQ. Tau-mediated neurodegeneration in Alzheimer's disease and related disorders. Nat Rev Neurosci. 2007;8:663–672. - PubMed
-
- Boekhoorn K, Terwel D, Biemans B, Borghgraef P, Wiegert O, Ramakers GJ, de Vos K, Krugers H, Tomiyama T, Mori H, Joels M, van Leuven F, Lucassen PJ. Improved long-term potentiation and memory in young tau-P301L transgenic mice before onset of hyperphosphorylation and tauopathy. J Neurosci. 2006;26:3514–3523. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

