Overexpression of Cu/Zn-superoxide dismutase and/or catalase accelerates benzo(a)pyrene detoxification by upregulation of the aryl hydrocarbon receptor in mouse endothelial cells
- PMID: 19666105
- PMCID: PMC2846758
- DOI: 10.1016/j.freeradbiomed.2009.08.001
Overexpression of Cu/Zn-superoxide dismutase and/or catalase accelerates benzo(a)pyrene detoxification by upregulation of the aryl hydrocarbon receptor in mouse endothelial cells
Abstract
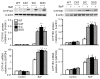
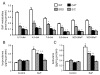
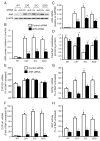
A reduction in endogenously generated reactive oxygen species in vivo delays benzo(a)pyrene (BaP)-accelerated atherosclerosis, as revealed in hypercholesterolemic mice overexpressing Cu/Zn-superoxide dismutase (SOD) and/or catalase. To understand the molecular events involved in this protective action, we studied the effects of Cu/Zn-SOD and/or catalase overexpression on BaP detoxification and on aryl hydrocarbon receptor (AhR) expression and its target gene expression in mouse aortic endothelial cells (MAECs). Our data demonstrate that overexpression of Cu/Zn-SOD and/or catalase leads to an 18- to 20-fold increase in the expression of AhR protein in MAECs. After BaP exposure, the amount of AhR binding to the cytochrome P450 (CYP) 1A1 promoter was significantly greater, and the concentrations of BaP reactive intermediates were significantly less in MAECs overexpressing Cu/Zn-SOD and/or catalase than in wild-type cells. In addition, the BaP-induced CYP1A1 and 1B1 protein levels and BaP-elevated glutathione S-transferase (GST) activity were significantly higher in these transgenic cells, in parallel with elevated GSTp1, CYP1A1, and CYP1B1 mRNA levels, compared to wild-type MAECs. Moreover, knockdown of AhR with RNA interference diminished the Cu/Zn-SOD and catalase enhancement of CYP1A1 expression, GST activity, and BaP detoxification. These data demonstrate that overexpression of Cu/Zn-SOD and/or catalase is associated with upregulation of AhR and its target genes, such as xenobiotic-metabolizing enzymes.
Figures




Similar articles
-
Overexpression of antioxidant enzymes upregulates aryl hydrocarbon receptor expression via increased Sp1 DNA-binding activity.Free Radic Biol Med. 2010 Aug 1;49(3):487-92. doi: 10.1016/j.freeradbiomed.2010.05.007. Epub 2010 May 15. Free Radic Biol Med. 2010. PMID: 20478378 Free PMC article.
-
Overexpression of Catalase Enhances Benzo(a)pyrene Detoxification in Endothelial Microsomes.PLoS One. 2016 Sep 8;11(9):e0162561. doi: 10.1371/journal.pone.0162561. eCollection 2016. PLoS One. 2016. PMID: 27607467 Free PMC article.
-
Inhibition of aryl hydrocarbon receptor transactivation and DNA adduct formation by CYP1 isoform-selective metabolic deactivation of benzo[a]pyrene.Toxicol Appl Pharmacol. 2008 Jul 15;230(2):135-43. doi: 10.1016/j.taap.2008.02.009. Epub 2008 Feb 21. Toxicol Appl Pharmacol. 2008. PMID: 18372000
-
Overexpression of antioxidant enzymes in ApoE-deficient mice suppresses benzo(a)pyrene-accelerated atherosclerosis.Atherosclerosis. 2009 Nov;207(1):51-8. doi: 10.1016/j.atherosclerosis.2009.03.052. Epub 2009 Apr 11. Atherosclerosis. 2009. PMID: 19409565 Free PMC article.
-
Oral benzo[a]pyrene: understanding pharmacokinetics, detoxication, and consequences--Cyp1 knockout mouse lines as a paradigm.Mol Pharmacol. 2013 Sep;84(3):304-13. doi: 10.1124/mol.113.086637. Epub 2013 Jun 12. Mol Pharmacol. 2013. PMID: 23761301 Free PMC article. Review.
Cited by
-
Comparative analysis of distinctive transcriptome profiles with biochemical evidence in bisphenol S- and benzo[a]pyrene-exposed liver tissues of the olive flounder Paralichthys olivaceus.PLoS One. 2018 May 1;13(5):e0196425. doi: 10.1371/journal.pone.0196425. eCollection 2018. PLoS One. 2018. PMID: 29715276 Free PMC article.
-
Regulation of the activity and expression of aryl hydrocarbon receptor by ethanol in mouse hepatic stellate cells.Alcohol Clin Exp Res. 2012 Nov;36(11):1873-81. doi: 10.1111/j.1530-0277.2012.01787.x. Epub 2012 Apr 6. Alcohol Clin Exp Res. 2012. PMID: 22486318 Free PMC article.
-
High-density lipoprotein-mediated transcellular cholesterol transport in mouse aortic endothelial cells.Biochem Biophys Res Commun. 2015 Sep 18;465(2):256-61. doi: 10.1016/j.bbrc.2015.08.011. Epub 2015 Aug 7. Biochem Biophys Res Commun. 2015. PMID: 26255968 Free PMC article.
-
AHR2 knockdown prevents PAH-mediated cardiac toxicity and XRE- and ARE-associated gene induction in zebrafish (Danio rerio).Toxicol Appl Pharmacol. 2011 Aug 1;254(3):280-7. doi: 10.1016/j.taap.2011.05.002. Epub 2011 May 10. Toxicol Appl Pharmacol. 2011. PMID: 21600235 Free PMC article.
-
A novel function of apolipoprotein E: upregulation of ATP-binding cassette transporter A1 expression.PLoS One. 2011;6(7):e21453. doi: 10.1371/journal.pone.0021453. Epub 2011 Jul 11. PLoS One. 2011. PMID: 21779326 Free PMC article.
References
-
- Abbott BD, Perdew GH, Birnbaum LS. Ah receptor in embryonic mouse palate and effects of TCDD on receptor expression. Toxicol Appl Pharmacol. 1994;126:16–25. - PubMed
-
- Bridges BA, Bowyer DE, Hansen ES, Penn A, Wakabayashi K. The possible involvement of somatic mutations in the development of atherosclerotic plaques. Mutat Res. 1990;239:143–148. - PubMed
-
- Burdick AD, Davis JW, II, Liu KJ, Hudson LG, Shi HL, Monske ML, Burchiel SW. Benzo(a)pyrene quinones increase cell proliferation, generate reactive oxygen species, and transactivate the epidermal growth factor receptor in breast epithelial cells. Cancer Res. 2003;63:7825–7833. - PubMed
-
- Davarinos NA, Pollenz RS. Aryl hydrocarbon receptor imported into the nucleus following ligand binding is rapidly degraded via the cytoplasmic proteasome following nuclear export. J Biol Chem. 1999;274:28708–28715. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

