A phase II study on safety and efficacy of high-dose N-acetylcysteine in patients with cystic fibrosis
- PMID: 19666395
- PMCID: PMC3352166
- DOI: 10.1186/2047-783x-14-8-352
A phase II study on safety and efficacy of high-dose N-acetylcysteine in patients with cystic fibrosis
Abstract
Objective: We conducted a single-centre, randomised, double-blinded, placebo-controlled phase II clinical study to test safety and efficacy of a 12-week therapy with low-dose (700 mg/daily) or high-dose (2800 mg/daily) of NAC.
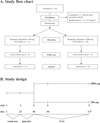
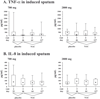
Methods: Twenty-one patients (DeltaF508 homo/heterozygous, FEV1>40% pred.) were included in the study. After a 3-weeks placebo run-in phase, 11 patients received low-dose NAC, and 10 patients received high-dose NAC. Outcomes included safety and clinical parameters, inflammatory (total leukocyte numbers, cell differentials, TNF-alpha, IL-8) measures in induced sputum, and concentrations of extracellular glutathione in induced sputum and blood.
Results: High-dose NAC was a well-tolerated and safe medication. High-dose NAC did not alter clinical or inflammatory parameters. However, extracellular glutathione in induced sputum tended to increase on high-dose NAC.
Conclusions: High-dose NAC is a well-tolerated and safe medication for a prolonged therapy of patients with CF with a potential to increase extracellular glutathione in CF airways.
Figures
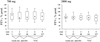

References
-
- Auerbach HS, Williams M, Kirkpatrick JA, Colten HR. Alternate-day prednisone reduces morbidity and improves pulmonary function in cystic fibrosis. Lancet. 1985;2(8457):686–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

