HIV-1 Gag processing intermediates trans-dominantly interfere with HIV-1 infectivity
- PMID: 19666477
- PMCID: PMC2785601
- DOI: 10.1074/jbc.M109.027144
HIV-1 Gag processing intermediates trans-dominantly interfere with HIV-1 infectivity
Abstract
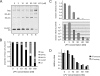
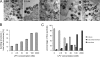
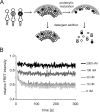
Protease inhibitors (PI) act by blocking human immunodeficiency virus (HIV) polyprotein processing, but there is no direct quantitative correlation between the degree of impairment of Gag processing and virion infectivity at low PI concentrations. To analyze the consequences of partial processing, virus particles were produced in the presence of limiting PI concentrations or by co-transfection of wild-type proviral plasmids with constructs carrying mutations in one or more cleavage sites. Low PI concentrations caused subtle changes in polyprotein processing associated with a pronounced reduction of particle infectivity. Dissection of individual stages of viral entry indicated a block in accumulation of reverse transcriptase products, whereas virus entry, enzymatic reverse transcriptase activity, and replication steps following reverse transcription were not affected. Co-expression of low amounts of partially processed forms of Gag together with wild-type HIV generally exerted a trans-dominant effect, which was most prominent for a construct carrying mutations at both cleavage sites flanking the CA domain. Interestingly, co-expression of low amounts of Gag mutated at the CA-SP1 cleavage site also affected processing activity at this site in the wild-type virus. The results indicate that low amounts (<5%) of Gag processing intermediates can display a trans-dominant effect on HIV particle maturation, with the maturation cleavage between CA and SP1 being of particular importance. These effects are likely to be important for the strong activity of PI at concentrations achieved in vivo and also bear relevance for the mechanism of action of the antiviral drug bevirimat.
Figures





Similar articles
-
HIV-1 protease inhibitor mutations affect the development of HIV-1 resistance to the maturation inhibitor bevirimat.Retrovirology. 2011 Aug 24;8:70. doi: 10.1186/1742-4690-8-70. Retrovirology. 2011. PMID: 21864346 Free PMC article.
-
Resistance to Second-Generation HIV-1 Maturation Inhibitors.J Virol. 2019 Mar 5;93(6):e02017-18. doi: 10.1128/JVI.02017-18. Print 2019 Mar 15. J Virol. 2019. PMID: 30567982 Free PMC article.
-
The capsid-spacer peptide 1 Gag processing intermediate is a dominant-negative inhibitor of HIV-1 maturation.Virology. 2010 Apr 25;400(1):137-44. doi: 10.1016/j.virol.2010.01.028. Epub 2010 Feb 20. Virology. 2010. PMID: 20172577 Free PMC article.
-
Human Immunodeficiency Virus Gag and protease: partners in resistance.Retrovirology. 2012 Aug 6;9:63. doi: 10.1186/1742-4690-9-63. Retrovirology. 2012. PMID: 22867298 Free PMC article. Review.
-
HIV-1 Maturation: Lessons Learned from Inhibitors.Viruses. 2020 Aug 26;12(9):940. doi: 10.3390/v12090940. Viruses. 2020. PMID: 32858867 Free PMC article. Review.
Cited by
-
Fusion of mature HIV-1 particles leads to complete release of a gag-GFP-based content marker and raises the intraviral pH.PLoS One. 2013 Aug 9;8(8):e71002. doi: 10.1371/journal.pone.0071002. eCollection 2013. PLoS One. 2013. PMID: 23951066 Free PMC article.
-
Structural analysis of HIV-1 maturation using cryo-electron tomography.PLoS Pathog. 2010 Nov 24;6(11):e1001215. doi: 10.1371/journal.ppat.1001215. PLoS Pathog. 2010. PMID: 21151640 Free PMC article.
-
Structural maturation of the matrix lattice is not required for HIV-1 particle infectivity.Sci Adv. 2025 May 9;11(19):eadv4356. doi: 10.1126/sciadv.adv4356. Epub 2025 May 9. Sci Adv. 2025. PMID: 40344051 Free PMC article.
-
Generation and characterization of a defective HIV-1 Virus as an immunogen for a therapeutic vaccine.PLoS One. 2012;7(11):e48848. doi: 10.1371/journal.pone.0048848. Epub 2012 Nov 7. PLoS One. 2012. PMID: 23144996 Free PMC article.
-
HIV-1 Protease Uses Bi-Specific S2/S2' Subsites to Optimize Cleavage of Two Classes of Target Sites.J Mol Biol. 2018 Dec 7;430(24):5182-5195. doi: 10.1016/j.jmb.2018.10.022. Epub 2018 Nov 7. J Mol Biol. 2018. PMID: 30414407 Free PMC article.
References
-
- Meek T. D., Lambert D. M., Dreyer G. B., Carr T. J., Tomaszek T. A., Jr., Moore M. L., Strickler J. E., Debouck C., Hyland L. J., Matthews T. J., et al. (1990) Nature 343, 90–92 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

