The cAMP binding protein Epac regulates cardiac myofilament function
- PMID: 19666481
- PMCID: PMC2729034
- DOI: 10.1073/pnas.0812536106
The cAMP binding protein Epac regulates cardiac myofilament function
Abstract
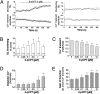
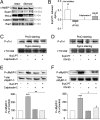
In the heart, cAMP is a key regulator of excitation-contraction coupling and its biological effects are mainly associated with the activity of protein kinase A (PKA). The aim of this study was to investigate the contribution of the cAMP-binding protein Epac (Exchange protein directly activated by cAMP) in the regulation of the contractile properties of rat ventricular cardiac myocytes. We report that both PKA and Epac increased cardiac sarcomere contraction but through opposite mechanisms. Differently from PKA, selective Epac activation by the cAMP analog 8-(4-chlorophenylthio)-2'-O-methyl-cAMP (8-pCPT) reduced Ca(2+) transient amplitude and increased cell shortening in intact cardiomyocytes and myofilament Ca(2+) sensitivity in permeabilized cardiomyocytes. Moreover, ventricular myocytes, which were infected in vivo with a constitutively active form of Epac, showed enhanced myofilament Ca(2+) sensitivity compared to control cells infected with green fluorescent protein (GFP) alone. At the molecular level, Epac increased phosphorylation of 2 key sarcomeric proteins, cardiac Troponin I (cTnI) and cardiac Myosin Binding Protein-C (cMyBP-C). The effects of Epac activation on myofilament Ca(2+) sensitivity and on cTnI and cMyBP-C phosphorylation were independent of PKA and were blocked by protein kinase C (PKC) and Ca(2+) calmodulin kinase II (CaMKII) inhibitors. Altogether these findings identify Epac as a new regulator of myofilament function.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415(6868):198–205. - PubMed
-
- Solaro RJ. Modulation of cardiac myofilament activity by protein phosphorylation. In: Page E, Fozzard H, Solaro RJ, editors. Handbook of Physiology. New York: Oxford Univ Press; 2001. pp. 264–300.
-
- Layland J, Solaro RJ, Shah AM. Regulation of cardiac contractile function by troponin I phosphorylation. Cardiovasc Res. 2005;66(1):12–21. - PubMed
-
- Kunst G, et al. Myosin binding protein C, a phosphorylation-dependent force regulator in muscle that controls the attachment of myosin heads by its interaction with myosin S2. Circ Res. 2000;86(1):51–58. - PubMed
-
- Cazorla O, et al. Length and protein kinase A modulations of myocytes in cardiac myosin binding protein C-deficient mice. Cardiovasc Res. 2006;69(2):370–380. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

