Structural analysis of substrate binding by the TatBC component of the twin-arginine protein transport system
- PMID: 19666509
- PMCID: PMC2718047
- DOI: 10.1073/pnas.0901566106
Structural analysis of substrate binding by the TatBC component of the twin-arginine protein transport system
Abstract
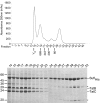
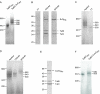
The Tat system transports folded proteins across the bacterial cytoplasmic membrane and the thylakoid membrane of plant chloroplasts. In Escherichia coli substrate proteins initially bind to the integral membrane TatBC complex which then recruits the protein TatA to effect translocation. Overproduction of TatBC and the substrate protein SufI in the absence of TatA led to the accumulation of TatBC-SufI complexes that could be purified using an affinity tag on the substrate. Three-dimensional structures of the TatBC-SufI complexes and unliganded TatBC were obtained by single-particle electron microscopy and random conical tilt reconstruction. Comparison of the structures shows that substrate molecules bind on the periphery of the TatBC complex and that substrate binding causes a significant reduction in diameter of the TatBC part of the complex. Although the TatBC complex contains multiple copies of the signal peptide-binding TatC protomer, purified TatBC-SufI complexes contain only 1 or 2 SufI molecules. Where 2 substrates are present in the TatBC-SufI complex, they are bound at adjacent sites. These observations imply that only certain TatC protomers within the complex interact with substrate or that there is a negative cooperativity of substrate binding. Similar TatBC-substrate complexes can be generated by an alternative in vitro reconstitution method and using a different substrate protein.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Berks BC, Palmer T, Sargent F. The Tat protein translocation pathway and its role in microbial physiology. Adv Microb Physiol. 2003;47:187–254. - PubMed
-
- Cline K, Theg SM. The Sec and Tat protein translocation pathways in chloroplasts. In: Dalbey RE, Koehler C, Tamanoi F, editors. The Enzymes, Molecular Machines Involved in Protein Transport across Cellular Membranes. Vol. XXV. San Diego, CA: Elsevier; 2007. pp. 455–485.
-
- Natale P, Brüser T, Driessen AJ. Sec- and Tat-mediated protein secretion across the bacterial cytoplasmic membrane—Distinct translocases and mechanisms. Biochim Biophys Acta. 2008;778:1735–1756. - PubMed
-
- Berks BC, Palmer T, Sargent F. Protein targeting by the bacterial twin-arginine translocation (Tat) pathway. Curr Opin Microbiol. 2005;8:174–181. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- D011140/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- C516144/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- 072681/Z/03/Z/WT_/Wellcome Trust/United Kingdom
- BB/C516179/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/D011140/2/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/C516144/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- G117/519/MRC_/Medical Research Council/United Kingdom
- BB/D011140/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/D004578/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- 079605/WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

