Peptides modulating conformational changes in secreted chaperones: from in silico design to preclinical proof of concept
- PMID: 19666568
- PMCID: PMC2728974
- DOI: 10.1073/pnas.0906514106
Peptides modulating conformational changes in secreted chaperones: from in silico design to preclinical proof of concept
Abstract
Blocking conformational changes in biologically active proteins holds therapeutic promise. Inspired by the susceptibility of viral entry to inhibition by synthetic peptides that block the formation of helix-helix interactions in viral envelope proteins, we developed a computational approach for predicting interacting helices. Using this approach, which combines correlated mutations analysis and Fourier transform, we designed peptides that target gp96 and clusterin, 2 secreted chaperones known to shift between inactive and active conformations. In human blood mononuclear cells, the gp96-derived peptide inhibited the production of TNFalpha, IL-1beta, IL-6, and IL-8 induced by endotoxin by >80%. When injected into mice, the peptide reduced circulating levels of endotoxin-induced TNFalpha, IL-6, and IFNgamma by >50%. The clusterin-derived peptide arrested proliferation of several neoplastic cell lines, and significantly enhanced the cytostatic activity of taxol in vitro and in a xenograft model of lung cancer. Also, the predicted mode of action of the active peptides was experimentally verified. Both peptides bound to their parent proteins, and their biological activity was abolished in the presence of the peptides corresponding to the counterpart helices. These data demonstrate a previously uncharacterized method for rational design of protein antagonists.
Conflict of interest statement
Conflict of interest statement: C.A.D. serves as a consultant to Compugen. To date, the total consulting fees have been less than $5,000. C.A.D. has no patents submitted or issued relevant to these studies. Neither C.A.D. nor his family hold any stocks or stock options in Compugen.
Figures

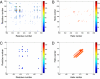

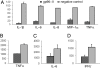

References
-
- Monod J, Wyman J, Changeux JP. On the nature of allosteric transitions: A plausible model. J Mol Biol. 1965;12:88–118. - PubMed
-
- Yin H, et al. Computational design of peptides that target transmembrane helices. Science. 2007;315:1817–1822. - PubMed
-
- Carr CM, Kim PS. A spring-loaded mechanism for the conformational change of influenza hemagglutinin. Cell. 1993;73:823–832. - PubMed
-
- Jones PL, Korte T, Blumenthal R. Conformational changes in cell surface HIV-1 envelope glycoproteins are triggered by cooperation between cell surface CD4 and co-receptors. J Biol Chem. 1998;273:404–409. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

