Silencing of tryptamine biosynthesis for production of nonnatural alkaloids in plant culture
- PMID: 19666570
- PMCID: PMC2728952
- DOI: 10.1073/pnas.0903393106
Silencing of tryptamine biosynthesis for production of nonnatural alkaloids in plant culture
Abstract
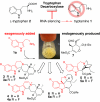
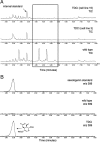
Natural products have long served as both a source and inspiration for pharmaceuticals. Modifying the structure of a natural product often improves the biological activity of the compound. Metabolic engineering strategies to ferment "unnatural" products have been enormously successful in microbial organisms. However, despite the importance of plant derived natural products, metabolic engineering strategies to yield unnatural products from complex, lengthy plant pathways have not been widely explored. Here, we show that RNA mediated suppression of tryptamine biosynthesis in Catharanthus roseus hairy root culture eliminates all production of monoterpene indole alkaloids, a class of natural products derived from two starting substrates, tryptamine and secologanin. To exploit this chemically silent background, we introduced an unnatural tryptamine analog to the production media and demonstrated that the silenced plant culture could produce a variety of novel products derived from this unnatural starting substrate. The novel alkaloids were not contaminated by the presence of the natural alkaloids normally present in C. roseus. Suppression of tryptamine biosynthesis therefore did not appear to adversely affect expression of downstream biosynthetic enzymes. Targeted suppression of substrate biosynthesis therefore appears to be a viable strategy for programming a plant alkaloid pathway to more effectively produce desirable unnatural products. Moreover, although tryptamine is widely found among plants, this silenced line demonstrates that tryptamine does not play an essential role in growth or development in C. roseus root culture. Silencing the biosynthesis of an early starting substrate enhances our ability to harness the rich diversity of plant based natural products.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
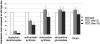
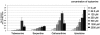

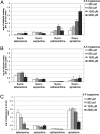
References
-
- Khosla C, Keasling JD. Metabolic engineering for drug discovery and development. Nat Rev Drug Disc. 2003;2:1019–1025. - PubMed
-
- Birch AJ. The biosynthesis of antibiotics. Pure Appl Chem. 1963;7:527–537.
-
- Weissman K. Mutasynthesis—uniting chemistry and genetics for drug discovery. Trends Biotechnol. 2007;25:139–142. - PubMed
-
- Weist S, Sussmuth RD. Mutational biosynthesis—a tool for the generation of structural diversity in the biosynthesis of antibiotics. Appl Microbiol Biotechnol. 2005;68:141–150. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

