Multiplexed imaging of surface enhanced Raman scattering nanotags in living mice using noninvasive Raman spectroscopy
- PMID: 19666578
- PMCID: PMC2726370
- DOI: 10.1073/pnas.0813327106
Multiplexed imaging of surface enhanced Raman scattering nanotags in living mice using noninvasive Raman spectroscopy
Abstract
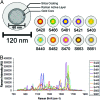
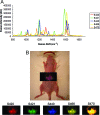
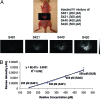
Raman spectroscopy is a newly developed, noninvasive preclinical imaging technique that offers picomolar sensitivity and multiplexing capabilities to the field of molecular imaging. In this study, we demonstrate the ability of Raman spectroscopy to separate the spectral fingerprints of up to 10 different types of surface enhanced Raman scattering (SERS) nanoparticles in a living mouse after s.c. injection. Based on these spectral results, we simultaneously injected the five most intense and spectrally unique SERS nanoparticles i.v. to image their natural accumulation in the liver. All five types of SERS nanoparticles were successfully identified and spectrally separated using our optimized noninvasive Raman imaging system. In addition, we were able to linearly correlate Raman signal with SERS concentration after injecting four spectrally unique SERS nanoparticles either s.c. (R(2) = 0.998) or i.v. (R(2) = 0.992). These results show great potential for multiplexed imaging in living subjects in cases in which several targeted SERS probes could offer better detection of multiple biomarkers associated with a specific disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Xing Y, et al. Bioconjugated quantum dots for multiplexed and quantitative immunohistochemistry. Nat Protoc. 2007;2:1152–1165. - PubMed
-
- Young SH, Rozengurt E. Qdot nanocrystal conjugates conjugated to bombesin or ANG II label the cognate G protein-coupled receptor in living cells. Am J Physiol. 2006;290:C728–C732. - PubMed
-
- Kim JH, et al. Nanoparticle probes with surface enhanced Raman spectroscopic tags for cellular cancer targeting. Anal Chem. 2006;78:6967–6973. - PubMed
-
- Chan WC, Maxwell DJ, Gao X, Bailey RE, Han M, Nie S. Luminescent quantum dots for multiplexed biological detection and imaging. Curr Opin Biotechnol. 2002;13:40–46. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

