The molecular basis for impaired hypoxia-induced VEGF expression in diabetic tissues
- PMID: 19666581
- PMCID: PMC2726398
- DOI: 10.1073/pnas.0906670106
The molecular basis for impaired hypoxia-induced VEGF expression in diabetic tissues
Abstract
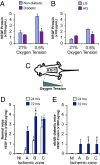
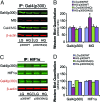
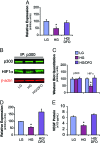
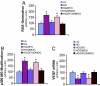
Diabetes is associated with poor outcomes following acute vascular occlusive events. This results in part from a failure to form adequate compensatory microvasculature in response to ischemia. Since vascular endothelial growth factor (VEGF) is an essential mediator of neovascularization, we examined whether hypoxic up-regulation of VEGF was impaired in diabetes. Both fibroblasts isolated from type 2 diabetic patients, and normal fibroblasts exposed chronically to high glucose, were defective in their capacity to up-regulate VEGF in response to hypoxia. In vivo, diabetic animals demonstrated an impaired ability to increase VEGF production in response to soft tissue ischemia. This resulted from a high glucose-induced decrease in transactivation by the transcription factor hypoxia-inducible factor-1alpha (HIF-1alpha), which mediates hypoxia-stimulated VEGF expression. Decreased HIF-1alpha functional activity was specifically caused by impaired HIF-1alpha binding to the coactivator p300. We identify covalent modification of p300 by the dicarbonyl metabolite methylglyoxal as being responsible for this decreased association. Administration of deferoxamine abrogated methylglyoxal conjugation, normalizing both HIF-1alpha/p300 interaction and transactivation by HIF-1alpha. In diabetic mice, deferoxamine promoted neovascularization and enhanced wound healing. These findings define molecular defects that underlie impaired VEGF production in diabetic tissues and offer a promising direction for therapeutic intervention.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Caro JJ, Ward AJ, O'Brien JA. Lifetime costs of complications resulting from type 2 diabetes in the U.S. Diabetes Care. 2002;25:476–481. - PubMed
-
- Norhammar A, et al. Glucose metabolism in patients with acute myocardial infarction and no previous diagnosis of diabetes mellitus: A prospective study. Lancet. 2002;359:2140–2144. - PubMed
-
- Abaci A, et al. Effect of diabetes mellitus on formation of coronary collateral vessels. Circulation. 1999;99:2239–2242. - PubMed
-
- Wilson PW. Diabetes mellitus and coronary heart disease. Am J Kidney Dis. 1998;32(Suppl 3):S89–S100. - PubMed
-
- Yla-Herttuala S, Rissanen TT, Vajanto I, Hartikainen J. Vascular endothelial growth factors: Biology and current status of clinical applications in cardiovascular medicine. J Am Coll Cardiol. 2007;49:1015–1026. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

