Epidemiological benefits of more-effective tuberculosis vaccines, drugs, and diagnostics
- PMID: 19666590
- PMCID: PMC2720405
- DOI: 10.1073/pnas.0901720106
Epidemiological benefits of more-effective tuberculosis vaccines, drugs, and diagnostics
Abstract
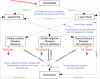
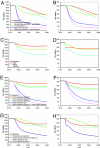
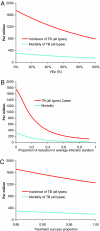
The Bill and Melinda Gates Foundation supports an ambitious portfolio of novel vaccines, drug regimens, and diagnostic tools for tuberculosis (TB). We elicited the expected efficacies and improvements of the novel interventions in discussions with the foundations managing their development. Using an age-structured mathematical model of TB, we explored the potential benefits of novel interventions under development and those not yet in the portfolio, focusing on the WHO Southeast Asia region. Neonatal vaccination with the portfolio vaccine decreases TB incidence by 39% to 52% by 2050. Drug regimens that shorten treatment duration and are efficacious against drug-resistant strains reduce incidence by 10-27%. New diagnostics reduce incidence by 13-42%. A triple combination of a portfolio vaccine, drug regimen, and diagnostics reduces incidence by 71%. A short mass vaccination catch-up campaign, not yet in the portfolio, to augment the triple combination, accelerates the decrease, preventing >30% more cases by 2050 than just the triple combination. New vaccines and drug regimens targeted at the vast reservoir of latently infected people, not in the portfolio, would reduce incidence by 37% and 82%, respectively. The combination of preventive latent therapy and a 2-month drug treatment regimen reduces incidence by 94%. Novel technologies in the pipeline would achieve substantial reductions in TB incidence, but not the Stop TB Partnership target for elimination. Elimination will require new delivery strategies, such as mass vaccination campaigns, and new products targeted at latently infected people.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Comment in
-
Parameters that influence the prediction of epidemiological benefits of more-effective tuberculosis vaccines, drugs, and diagnosis.Proc Natl Acad Sci U S A. 2009 Nov 17;106(46):E129; author reply E130. doi: 10.1073/pnas.0911020106. Epub 2009 Oct 26. Proc Natl Acad Sci U S A. 2009. PMID: 19858487 Free PMC article. No abstract available.
References
-
- Skeiky YA, Sadoff JC. Advances in tuberculosis vaccine strategies. Nat Rev Microbiol. 2006;4:469–476. - PubMed
-
- TB Alliance. Confronting TB: What It Takes, 2008 Annual Report. New York: Global Alliance for TB Drug Development; 2008.
-
- Keeler E, et al. Reducing the global burden of tuberculosis: the contribution of improved diagnostics. Nature. 2006;444(Suppl 1):49–57. - PubMed
-
- Raviglione MC, Uplekar MW. WHO's new stop TB strategy. Lancet. 2006;367:952–955. - PubMed
-
- WHO. WHO Report 2008. Geneva: WHO; 2008. Global tuberculosis control: Surveillance, planning, financing.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

