The origin of malignant malaria
- PMID: 19666593
- PMCID: PMC2720412
- DOI: 10.1073/pnas.0907740106
The origin of malignant malaria
Abstract
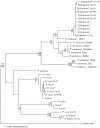
Plasmodium falciparum, the causative agent of malignant malaria, is among the most severe human infectious diseases. The closest known relative of P. falciparum is a chimpanzee parasite, Plasmodium reichenowi, of which one single isolate was previously known. The co-speciation hypothesis suggests that both parasites evolved separately from a common ancestor over the last 5-7 million years, in parallel with the divergence of their hosts, the hominin and chimpanzee lineages. Genetic analysis of eight new isolates of P. reichenowi, from wild and wild-born captive chimpanzees in Cameroon and Côte d'Ivoire, shows that P. reichenowi is a geographically widespread and genetically diverse chimpanzee parasite. The genetic lineage comprising the totality of global P. falciparum is fully included within the much broader genetic diversity of P. reichenowi. This finding is inconsistent with the co-speciation hypothesis. Phylogenetic analysis indicates that all extant P. falciparum populations originated from P. reichenowi, likely by a single host transfer, which may have occurred as early as 2-3 million years ago, or as recently as 10,000 years ago. The evolutionary history of this relationship may be explained by two critical genetic mutations. First, inactivation of the CMAH gene in the human lineage rendered human ancestors unable to generate the sialic acid Neu5Gc from its precursor Neu5Ac, and likely made humans resistant to P. reichenowi. More recently, mutations in the dominant invasion receptor EBA 175 in the P. falciparum lineage provided the parasite with preference for the overabundant Neu5Ac precursor, accounting for its extreme human pathogenicity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


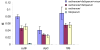
Comment in
-
Human-specific evolution of sialic acid targets: explaining the malignant malaria mystery?Proc Natl Acad Sci U S A. 2009 Sep 1;106(35):14739-40. doi: 10.1073/pnas.0908196106. Epub 2009 Aug 26. Proc Natl Acad Sci U S A. 2009. PMID: 19717444 Free PMC article. No abstract available.
References
-
- Boyd MF. Malariology: A comprehensive survey of all aspects of this group of diseases from a global standpoint. Philadelphia: Saunders; 1949. pp. 1–47.
-
- Escalante AA, Barrio E, Ayala FJ. Evolutionary origin of human and primate malarias: Evidence from the circumsporozoite protein gene. Mol Biol Evol. 1995;12:616–626. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

