Human BAHD1 promotes heterochromatic gene silencing
- PMID: 19666599
- PMCID: PMC2728979
- DOI: 10.1073/pnas.0901259106
Human BAHD1 promotes heterochromatic gene silencing
Abstract
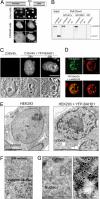
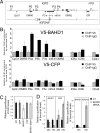
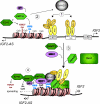
Gene silencing via heterochromatin formation plays a major role in cell differentiation and maintenance of homeostasis. Here we report the identification and characterization of a novel heterochromatinization factor in vertebrates, bromo adjacent homology domain-containing protein 1 (BAHD1). This nuclear protein interacts with HP1, MBD1, HDAC5, and several transcription factors. Through electron and immunofluorescence microscopy studies, we show that BAHD1 overexpression directs HP1 to specific nuclear sites and promotes the formation of large heterochromatic domains, which lack acetyl histone H4 and are enriched in H3 trimethylated at lysine 27 (H3K27me3). Furthermore, ectopically expressed BAHD1 colocalizes with the heterochromatic inactive X chromosome (Xi). The BAH domain is required for BAHD1 colocalization with H3K27me3, but not with the Xi chromosome. As highlighted by whole genome microarray analysis of BAHD1 knockdown cells, BAHD1 represses several proliferation and survival genes, in particular the insulin-like growth factor II gene (IGF2). When overexpressed, BAHD1 specifically binds the CpG-rich P3 promoter of IGF2, which increases MBD1 and HDAC5 targeting at this locus. This region contains DNA-binding sequences for the transcription factor SP1, with which BAHD1 coimmunoprecipitates. Collectively, these findings provide evidence that BAHD1 acts as a silencer by recruiting at specific promoters a set of proteins that coordinate heterochromatin assembly.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Trojer P, Reinberg D. Facultative heterochromatin: Is there a distinctive molecular signature? Mol Cell. 2007;28:1–13. - PubMed
-
- Feinberg AP. Phenotypic plasticity and the epigenetics of human disease. Nature. 2007;447:433–440. - PubMed
-
- Wang GG, Allis CD, Chi P. Chromatin remodeling and cancer, part I: Covalent histone modifications. Trends Mol Med. 2007;13:363–372. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

