Abscisic acid represses growth of the Arabidopsis embryonic axis after germination by enhancing auxin signaling
- PMID: 19666738
- PMCID: PMC2751952
- DOI: 10.1105/tpc.109.067702
Abscisic acid represses growth of the Arabidopsis embryonic axis after germination by enhancing auxin signaling
Abstract
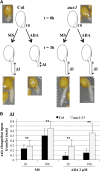
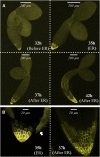
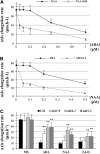
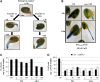
Under unfavorable environmental conditions, the stress phytohormone ABA inhibits the developmental transition from an embryo in a dry seed into a young seedling. We developed a genetic screen to isolate Arabidopsis thaliana mutants whose early seedling development is resistant to ABA. Here, we report the identification of a recessive mutation in AUXIN RESISTANT1 (AUX1), encoding a cellular auxin influx carrier. Although auxin is a major morphogenesis hormone in plants, little is known about ABA-auxin interactions during early seedling growth. We show that aux1 and pin2 mutants are insensitive to ABA-dependent repression of embryonic axis (hypocotyl and radicle) elongation. Genetic and physiological experiments show that this involves auxin transport to the embryonic axis elongation zone, where ABA enhances the activity of an auxin-responsive promoter. We propose that ABA represses embryonic axis elongation by potentiating auxin signaling in its elongation zone. This involves repression of the AUXIN INDUCIBLE (Aux/IAA) gene AXR2/IAA7, encoding a key component of ABA- and auxin-dependent responses during postgerminative growth.
Figures




Similar articles
-
Arabidopsis zinc-finger protein 2 is a negative regulator of ABA signaling during seed germination.J Plant Physiol. 2010 Nov 1;167(16):1418-21. doi: 10.1016/j.jplph.2010.05.010. Epub 2010 Jul 8. J Plant Physiol. 2010. PMID: 20619483
-
Re-induction of the cell cycle in the Arabidopsis post-embryonic root meristem is ABA-insensitive, GA-dependent and repressed by KRP6.Sci Rep. 2016 Mar 29;6:23586. doi: 10.1038/srep23586. Sci Rep. 2016. PMID: 27021201 Free PMC article.
-
Arabidopsis WRKY6 Transcription Factor Acts as a Positive Regulator of Abscisic Acid Signaling during Seed Germination and Early Seedling Development.PLoS Genet. 2016 Feb 1;12(2):e1005833. doi: 10.1371/journal.pgen.1005833. eCollection 2016 Feb. PLoS Genet. 2016. PMID: 26829043 Free PMC article.
-
Abscisic acid signaling in seeds and seedlings.Plant Cell. 2002;14 Suppl(Suppl):S15-45. doi: 10.1105/tpc.010441. Plant Cell. 2002. PMID: 12045268 Free PMC article. Review. No abstract available.
-
Multifaceted Signaling Networks Mediated by Abscisic Acid Insensitive 4.Plant Commun. 2020 Mar 7;1(3):100040. doi: 10.1016/j.xplc.2020.100040. eCollection 2020 May 11. Plant Commun. 2020. PMID: 33367237 Free PMC article. Review.
Cited by
-
Genome-wide identification of the auxin response factor (ARF) gene family in Magnolia sieboldii and functional analysis of MsARF5.Front Plant Sci. 2022 Oct 5;13:958816. doi: 10.3389/fpls.2022.958816. eCollection 2022. Front Plant Sci. 2022. PMID: 36275560 Free PMC article.
-
Abscisic Acid and Abiotic Stress Tolerance in Crop Plants.Front Plant Sci. 2016 May 4;7:571. doi: 10.3389/fpls.2016.00571. eCollection 2016. Front Plant Sci. 2016. PMID: 27200044 Free PMC article. Review.
-
HRS1 acts as a negative regulator of abscisic acid signaling to promote timely germination of Arabidopsis seeds.PLoS One. 2012;7(4):e35764. doi: 10.1371/journal.pone.0035764. Epub 2012 Apr 24. PLoS One. 2012. PMID: 22545134 Free PMC article.
-
YUCCA4 overexpression modulates auxin biosynthesis and transport and influences plant growth and development via crosstalk with abscisic acid in Arabidopsis thaliana.Genet Mol Biol. 2020 Feb 17;43(1):e20190221. doi: 10.1590/1678-4685-GMB-2019-0221. eCollection 2020. Genet Mol Biol. 2020. PMID: 32105289 Free PMC article.
-
IAA-Ala Resistant3, an evolutionarily conserved target of miR167, mediates Arabidopsis root architecture changes during high osmotic stress.Plant Cell. 2012 Sep;24(9):3590-602. doi: 10.1105/tpc.112.097006. Epub 2012 Sep 7. Plant Cell. 2012. PMID: 22960911 Free PMC article.
References
-
- Abel, S., Nguyen, M.D., and Theologis, A. (1995). The PS-IAA4/5-like family of early auxin-inducible mRNAs in Arabidopsis thaliana. J. Mol. Biol. 251: 533–549. - PubMed
-
- Alonso, J.M., et al. (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657. - PubMed
-
- Benjamins, R., and Scheres, B. (2008). Auxin: The looping star in plant development. Annu. Rev. Plant Biol. 59: 443–465. - PubMed
-
- Bennett, M.J., Marchant, A., Green, H.G., May, S.T., Ward, S.P., Millner, P.A., Walker, A.R., Schulz, B., and Feldmann, K.A. (1996). Arabidopsis AUX1 gene: A permease-like regulator of root gravitropism. Science 273: 948–950. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

