MARCKS modulates radial progenitor placement, proliferation and organization in the developing cerebral cortex
- PMID: 19666823
- PMCID: PMC2723068
- DOI: 10.1242/dev.036616
MARCKS modulates radial progenitor placement, proliferation and organization in the developing cerebral cortex
Abstract
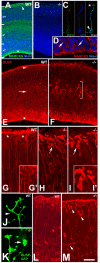
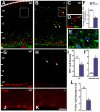
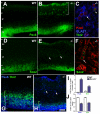
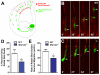
The radial glial cells serve as neural progenitors and as a migratory guide for newborn neurons in the developing cerebral cortex. These functions require appropriate organization and proliferation of the polarized radial glial scaffold. Here, we demonstrate in mice that the myristoylated alanine-rich C-kinase substrate protein (MARCKS), a prominent cellular substrate for PKC, modulates radial glial placement and expansion. Loss of MARCKS results in ectopic collection of mitotically active radial progenitors away from the ventricular zone (VZ) in the upper cerebral wall. Apical restriction of key polarity complexes [CDC42, beta-catenin (CTNNB1), N-cadherin (CDH2), myosin IIB (MYOIIB), aPKCzeta, LGL, PAR3, pericentrin, PROM1] is lost. Furthermore, the radial glial scaffold in Marcks null cortex is compromised, with discontinuous, non-radial processes apparent throughout the cerebral wall and deformed, bulbous, unbranched end-feet at the basal ends. Further, the density of radial processes within the cerebral cortex is reduced. These deficits in radial glial development culminate in aberrant positioning of neurons and disrupted cortical lamination. Genetic rescue experiments demonstrate, surprisingly, that phosphorylation of MARCKS by PKC is not essential for the role of MARCKS in radial glial cell development. By contrast, the myristoylation domain of MARCKS needed for membrane association is essential for MARCKS function in radial glia. The membrane-associated targeting of MARCKS and the resultant polarized distribution of signaling complexes essential for apicobasal polarity may constitute a critical event in the appropriate placement, proliferation and organization of polarized radial glial scaffold in the developing cerebral cortex.
Figures




Similar articles
-
Cdc42 and Gsk3 modulate the dynamics of radial glial growth, inter-radial glial interactions and polarity in the developing cerebral cortex.Development. 2010 Dec;137(23):4101-10. doi: 10.1242/dev.048637. Development. 2010. PMID: 21062867 Free PMC article.
-
The adenomatous polyposis coli protein is an essential regulator of radial glial polarity and construction of the cerebral cortex.Neuron. 2009 Jan 15;61(1):42-56. doi: 10.1016/j.neuron.2008.10.053. Neuron. 2009. PMID: 19146812 Free PMC article.
-
Arl13b-regulated cilia activities are essential for polarized radial glial scaffold formation.Nat Neurosci. 2013 Aug;16(8):1000-7. doi: 10.1038/nn.3451. Epub 2013 Jun 30. Nat Neurosci. 2013. PMID: 23817546 Free PMC article.
-
Identification of myristoylated alanine-rich C kinase substrate (MARCKS) in astrocytes.Front Biosci. 2005 Jan 1;10:160-5. doi: 10.2741/1517. Print 2005 Jan 1. Front Biosci. 2005. PMID: 15574358 Review.
-
Radial glial cells as neuronal precursors: a new perspective on the correlation of morphology and lineage restriction in the developing cerebral cortex of mice.Brain Res Bull. 2002 Apr;57(6):777-88. doi: 10.1016/s0361-9230(01)00777-8. Brain Res Bull. 2002. PMID: 12031274 Review.
Cited by
-
MPP3 is required for maintenance of the apical junctional complex, neuronal migration, and stratification in the developing cortex.J Neurosci. 2013 May 8;33(19):8518-27. doi: 10.1523/JNEUROSCI.5627-12.2013. J Neurosci. 2013. PMID: 23658188 Free PMC article.
-
Spatiotemporal Regulation of Rho GTPases in Neuronal Migration.Cells. 2019 Jun 10;8(6):568. doi: 10.3390/cells8060568. Cells. 2019. PMID: 31185627 Free PMC article. Review.
-
Morphological and functional aspects of progenitors perturbed in cortical malformations.Front Cell Neurosci. 2015 Feb 12;9:30. doi: 10.3389/fncel.2015.00030. eCollection 2015. Front Cell Neurosci. 2015. PMID: 25729350 Free PMC article. Review.
-
Beta-catenin signaling negatively regulates intermediate progenitor population numbers in the developing cortex.PLoS One. 2010 Aug 25;5(8):e12376. doi: 10.1371/journal.pone.0012376. PLoS One. 2010. PMID: 20811503 Free PMC article.
-
Altered cholesterol biosynthesis causes precocious neurogenesis in the developing mouse forebrain.Neurobiol Dis. 2016 Jul;91:69-82. doi: 10.1016/j.nbd.2016.02.017. Epub 2016 Feb 24. Neurobiol Dis. 2016. PMID: 26921468 Free PMC article.
References
-
- Aderem, A. (1992a). The MARCKS brothers: a family of protein kinase C substrates. Cell 71, 713-716. - PubMed
-
- Aderem, A. (1992b). Signal transduction and the actin cytoskeleton: the roles of MARCKS and profilin. Trends Biochem. Sci. 17, 438-443. - PubMed
-
- Ayala, R., Shu, T. and Tsai, L. H. (2007). Trekking across the brain: the journey of neuronal migration. Cell 128, 29-43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

