Phasic contractions of rat mesenteric lymphatics increase basal and phasic nitric oxide generation in vivo
- PMID: 19666850
- PMCID: PMC2770767
- DOI: 10.1152/ajpheart.00039.2009
Phasic contractions of rat mesenteric lymphatics increase basal and phasic nitric oxide generation in vivo
Abstract
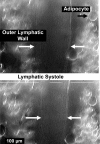
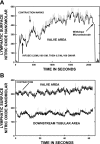
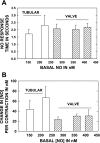
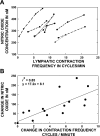
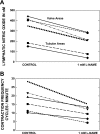
Multiple investigators have shown interdependence of lymphatic contractions on nitric oxide (NO) activity by pharmacological and traumatic suppression of endothelial NO synthase (eNOS). We demonstrated that lymphatic diastolic relaxation is particularly sensitive to NO from the lymphatic endothelium. The predicted mechanism is shear forces produced by the lymph flow during phasic pumping, activating eNOS in the lymphatic endothelium to produce NO. We measured [NO] during phasic contractions using microelectrodes on in situ mesenteric lymphatics in anesthetized rats under basal conditions and with an intravenous saline bolus (0.5 ml/100 g) or infusion (0.5 ml x 100 g(-1) x h(-1)). Under basal conditions, [NO] measured on the tubular portions of the lymphatics was approximately 200-250 nM, slightly higher than in the adjacent adipocyte microvasculature, whereas [NO] measured on the lymphatic bulb surface was approximately 400 nM. Immunohistochemistry of eNOS in isolated lympathics indicated a much greater expression in the lymph valves and surrounding bulb area than in the tubular regions. During phasic lymphatic contractions, the valve and tubular [NO] increased with each contraction, and during intravenous saline infusion, [NO] increased in proportion to the contraction frequency and, presumably, lymph flow. The partial blockade of eNOS over approximately 1 cm length with N(omega)-nitro-L-arginine methyl ester lowered the [NO]. These in vivo data document for the first time that both valvular and tubular lymphatic segments increase NO generation during each phasic contraction and that [NO] summated with increased contraction frequency. The combined data predict regional variations in eNOS and [NO] in the tubular and valve areas, plus the summated NO responses dependent on contraction frequency provide for a complex relaxation mechanism involving NO.
Figures


References
-
- Bauser-Heaton HD, Bohlen HG. Cerebral microvascular dilation during hypotension and decreased oxygen tension: a role for nNOS. Am J Physiol Heart Circ Physiol 293: H2193–H2201, 2007 - PubMed
-
- Bauser-Heaton HD, Song J, Bohlen HG. Cerebral microvascular nNOS responds to lowered oxygen tension through a bumetanide-sensitive cotransporter and sodium-calcium exchanger. Am J Physiol Heart Circ Physiol 294: H2166–H2173, 2008 - PubMed
-
- Benoit JN, Zawieja DC. Effects of f-Met-Leu-Phe-induced inflammation on intestinal lymph flow and lymphatic pump behavior. Am J Physiol Gastrointest Liver Physiol 262: G199–G202, 1992 - PubMed
-
- Benoit JN, Zawieja DC, Goodman AH, Granger HJ. Characterization of intact mesenteric lymphatic pump and its responsiveness to acute edemagenic stress. Am J Physiol Heart Circ Physiol 257: H2059–H2069, 1989 - PubMed
-
- Bohlen HG. Intestinal mucosal oxygenation influences absorptive hyperemia. Am J Physiol Heart Circ Physiol 239: H489–H493, 1980 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

