Severe loss of invariant NKT cells exhibiting anti-HTLV-1 activity in patients with HTLV-1-associated disorders
- PMID: 19666871
- PMCID: PMC2759648
- DOI: 10.1182/blood-2009-02-203042
Severe loss of invariant NKT cells exhibiting anti-HTLV-1 activity in patients with HTLV-1-associated disorders
Abstract
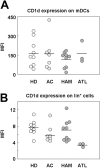
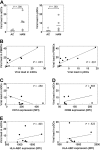
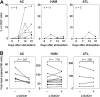
Invariant natural killer T (iNKT) cells are unique T cells that regulate the immune response to microbes, cancers, and autoimmunity. We assessed the characteristics of iNKT cells from persons infected with human T-lymphotropic virus type 1 (HTLV-1). Whereas most infected persons remain asymptomatic carriers (ACs) throughout their lives, a small proportion, usually with high equilibrium proviral loads,develop 2 diseases: HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) and adult T-cell leukemia (ATL). We demonstrated that the frequency of iNKT, NK, and dendritic cells in the peripheral blood of HAM/TSP and ATL patients is decreased. We also observed an inverse correlation between the iNKT cell frequency and the HTLV-1 proviral load in the peripheral blood of infected persons. Notably, in vitro stimulation of peripheral blood cells with alpha-galactosylceramide led to an increase in the iNKT cell number and a subsequent decrease in the HTLV-1-infected T-cell number in samples from ACs but not HAM/TSP or ATL patients. Our results suggest that iNKT cells contribute to the immune defense against HTLV-1, and iNKT-cell depletion plays an important role in the pathogenesis of HAM/TSP and ATL. Therefore, iNKT cell-based immunotherapy may be an effective strategy for preventing these HTLV-1-associated disorders.
Figures



References
-
- Uchiyama T, Yodoi J, Sagawa K, Takatsuki K, Uchino H. Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood. 1977;50(3):481–492. - PubMed
-
- Gessain A, Barin F, Vernant JC, et al. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet. 1985;2(8452):407–410. - PubMed
-
- Osame M, Usuku K, Izumo S, et al. HTLV-I associated myelopathy, a new clinical entity. Lancet. 1986;1(8488):1031–1032. - PubMed
-
- Nagai M, Usuku K, Matsumoto W, et al. Analysis of HTLV-I proviral load in 202 HAM/TSP patients and 243 asymptomatic HTLV-I carriers: high proviral load strongly predisposes to HAM/TSP. J Neurovirol. 1998;4(6):586–593. - PubMed
-
- Nagai M, Yamano Y, Brennan MB, Mora CA, Jacobson S. Increased HTLV-I proviral load and preferential expansion of HTLV-I Tax-specific CD8+ T cells in cerebrospinal fluid from patients with HAM/TSP. Ann Neurol. 2001;50(6):807–812. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

