Cytolytic T cells induce ceramide-rich platforms in target cell membranes to initiate graft-versus-host disease
- PMID: 19666872
- PMCID: PMC2766684
- DOI: 10.1182/blood-2008-11-191148
Cytolytic T cells induce ceramide-rich platforms in target cell membranes to initiate graft-versus-host disease
Abstract
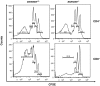
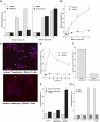
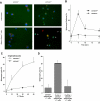
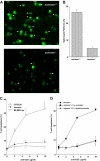
Alloreactive donor cytolytic T lymphocytes play a critical role in pathophysiology of acute graft-versus-host disease (GVHD). As GVHD progression involves tumor necrosis factor superfamily receptor activation, and as apoptotic signaling for some tumor necrosis factor superfamily receptors might involve acid sphingomyelinase (ASMase)-mediated ceramide generation, we hypothesized that ASMase deletion would ameliorate GVHD. Using clinically relevant mouse models of acute GVHD in which allogeneic bone marrow and T cells were transplanted into asmase+/+ and asmase(-/-) hosts, we identify host ASMase as critical for full-blown GVHD. Lack of host ASMase reduced the acute inflammatory phase of GVHD, attenuating cytokine storm, CD8+ T-cell proliferation/activation, and apoptosis of relevant graft-versus-host target cells (hepatocytes, intestinal, and skin cells). Organ injury was diminished in asmase(-/-) hosts, and morbidity and mortality improved at 90 days after transplantation. Resistance to cytolytic T lymphocyte-induced apoptosis was found at the target cell membrane if hepatocytes lack ASMase, as hepatocyte apoptosis required target cell ceramide generation for formation of ceramide-rich macrodomains, sites concentrating proapoptotic Fas. These studies indicate a requirement for target cell ASMase in evolution of GVHD in liver, small intestines, and skin and provide potential new targets for disease management.
Figures



Comment in
-
Acid sphingomyelinase deficiency does not protect from graft-versus-host disease in transplant recipients with Niemann-Pick disease.Blood. 2010 Jan 14;115(2):434-5. doi: 10.1182/blood-2009-09-245167. Blood. 2010. PMID: 20075175 No abstract available.
Similar articles
-
Acid sphingomyelinase deficiency does not protect from graft-versus-host disease in transplant recipients with Niemann-Pick disease.Blood. 2010 Jan 14;115(2):434-5. doi: 10.1182/blood-2009-09-245167. Blood. 2010. PMID: 20075175 No abstract available.
-
Fas-deficient lpr mice are more susceptible to graft-versus-host disease.J Immunol. 2000 Jan 1;164(1):469-80. doi: 10.4049/jimmunol.164.1.469. J Immunol. 2000. PMID: 10605044
-
Paradoxical effects of interleukin-18 on the severity of acute graft-versus-host disease mediated by CD4+ and CD8+ T-cell subsets after experimental allogeneic bone marrow transplantation.Blood. 2004 Nov 15;104(10):3393-9. doi: 10.1182/blood-2004-02-0763. Epub 2004 Jul 27. Blood. 2004. PMID: 15280194
-
Acute graft-vs-host disease: pathobiology and management.Exp Hematol. 2001 Mar;29(3):259-77. doi: 10.1016/s0301-472x(00)00677-9. Exp Hematol. 2001. PMID: 11274753 Review.
-
Th2 and Tc2 cells in the regulation of GVHD, GVL, and graft rejection: considerations for the allogeneic transplantation therapy of leukemia and lymphoma.Leuk Lymphoma. 2000 Jul;38(3-4):221-34. doi: 10.3109/10428190009087014. Leuk Lymphoma. 2000. PMID: 10830730 Review.
Cited by
-
Using ASMase knockout mice to model human diseases.Handb Exp Pharmacol. 2013;216(216):29-54. doi: 10.1007/978-3-7091-1511-4_2. Handb Exp Pharmacol. 2013. PMID: 23563650 Free PMC article. Review.
-
Ceramide-rich platforms in transmembrane signaling.FEBS Lett. 2010 May 3;584(9):1728-40. doi: 10.1016/j.febslet.2010.02.026. Epub 2010 Feb 20. FEBS Lett. 2010. PMID: 20178791 Free PMC article. Review.
-
Treatment with agonistic DR3 antibody results in expansion of donor Tregs and reduced graft-versus-host disease.Blood. 2015 Jul 23;126(4):546-57. doi: 10.1182/blood-2015-04-637587. Epub 2015 Jun 10. Blood. 2015. PMID: 26063163 Free PMC article.
-
Ceramide synthase 6 impacts T-cell allogeneic response and graft-versus-host disease through regulating N-RAS/ERK pathway.Leukemia. 2022 Jul;36(7):1907-1915. doi: 10.1038/s41375-022-01581-6. Epub 2022 May 5. Leukemia. 2022. PMID: 35513703 Free PMC article.
-
Sphingomyelin Breakdown in T Cells: Role of Membrane Compartmentalization in T Cell Signaling and Interference by a Pathogen.Front Cell Dev Biol. 2019 Aug 13;7:152. doi: 10.3389/fcell.2019.00152. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31457008 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

