Neuraminidase inhibitors for treatment and prophylaxis of influenza in children: systematic review and meta-analysis of randomised controlled trials
- PMID: 19666987
- PMCID: PMC2724601
- DOI: 10.1136/bmj.b3172
Neuraminidase inhibitors for treatment and prophylaxis of influenza in children: systematic review and meta-analysis of randomised controlled trials
Abstract
Objective: To assess the effects of the neuraminidase inhibitors oseltamivir and zanamivir in treatment of children with seasonal influenza and prevention of transmission to children in households.
Design: Systematic review and meta-analysis of data from published and unpublished randomised controlled trials.
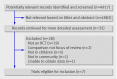
Data sources: Medline and Embase to June 2009, trial registries, and manufacturers and authors of relevant studies. Review methods Eligible studies were randomised controlled trials of neuraminidase inhibitors in children aged </=12 in the community (that is, not admitted to hospital) with confirmed or clinically suspected influenza. Primary outcome measures were time to resolution of illness and incidence of influenza in children living in households with index cases of influenza.
Results: We identified four randomised trials of treatment of influenza (two with oseltamivir, two with zanamivir) involving 1766 children (1243 with confirmed influenza, of whom 55-69% had influenza A), and three randomised trials for postexposure prophylaxis (one with oseltamivir, two with zanamivir) involving 863 children; none of these trials tested efficacy with the current pandemic strain. Treatment trials showed reductions in median time to resolution of symptoms or return to normal activities, or both, of 0.5-1.5 days, which were significant in only two trials. A 10 day course of postexposure prophylaxis with zanamivir or oseltamivir resulted in an 8% (95% confidence interval 5% to 12%) decrease in the incidence of symptomatic influenza. Based on only one trial, oseltamivir did not reduce asthma exacerbations or improve peak flow in children with asthma. Treatment was not associated with reduction in overall use of antibiotics (risk difference -0.30, -0.13 to 0.01). Zanamivir was well tolerated, but oseltamivir was associated with an increased risk of vomiting (0.05, 0.02 to 0.09, number needed to harm=20).
Conclusions: Neuraminidase inhibitors provide a small benefit by shortening the duration of illness in children with seasonal influenza and reducing household transmission. They have little effect on asthma exacerbations or the use of antibiotics. Their effects on the incidence of serious complications, and on the current A/H1N1 influenza strain remain to be determined.
Conflict of interest statement
Competing interests: None declared.
Figures
Comment in
-
A/H1N1 flu pandemic. Antiviral drugs: distinguish treatment from prophylaxis.BMJ. 2009 Sep 8;339:b3620. doi: 10.1136/bmj.b3620. BMJ. 2009. PMID: 19737833 No abstract available.
-
Neuraminidase inhibitors beneficial for treatment and prevention of influenza in children.J Pediatr. 2010 Mar;156(3):510-1. doi: 10.1016/j.jpeds.2009.11.070. J Pediatr. 2010. PMID: 20176198 No abstract available.
-
Neuraminidase inhibitors produce a small reduction in duration of seasonal influenza in children and reduce transmission in affected households, but effects on serious complications unclear.Evid Based Med. 2010 Feb;15(1):20-1. doi: 10.1136/ebm.15.1.20. Evid Based Med. 2010. PMID: 20176878 No abstract available.
-
Neuraminidase inhibitors produce a small reduction in duration of seasonal flu in children and reduce transmission in affected households, but effects on serious complications are unclear.Evid Based Nurs. 2010 Feb;13(1):23-4. doi: 10.1136/ebn1024. Evid Based Nurs. 2010. PMID: 20179067 No abstract available.
References
-
- Glezen WP, Couch RB. Interpandemic influenza in the Houston area, 1974-76. N Engld J Med 1978;298: 587-92. - PubMed
-
- Poehling KA, Edwards KM, Weinberg GA, Szilagyi P, Staat MA, Iwanr MK, et al. New Vaccine Surveillance Network. The under recognized burden of influenza in young children. N Engl J Med 2006;355:31-40. - PubMed
-
- Neuzil KM, Zhu Y, Griffin MR, Edwards KM, Thompson JM, Tollefson SJ, et al. Burden of interpandemic influenza in children younger than 5 years: a 25-year prospective study. J Infect Dis 2002;185:147-52. - PubMed
-
- Bhat N, Wright JG, Broder KR, Murray AL, Greenberg ME, Glover MJ, et al. Influenza-associated deaths among children in the United States, 2003-2004. N Engl J Med 2005;353:2559-67. - PubMed
-
- Health Protection Agency. HPA weekly national influenza report: week 27. 2009 July 1. www.hpa.org.uk/web/HPAweb&HPAwebStandard/HPAweb_C/1243928258754.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical