Cytotoxicity and secretion of gamma interferon are carried out by distinct CD8 T cells during Mycobacterium tuberculosis infection
- PMID: 19667047
- PMCID: PMC2747936
- DOI: 10.1128/IAI.00415-09
Cytotoxicity and secretion of gamma interferon are carried out by distinct CD8 T cells during Mycobacterium tuberculosis infection
Abstract
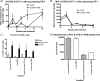
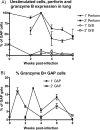
The host immune response is generally sufficient to contain Mycobacterium tuberculosis infection. It does not, however, efficiently prevent subsequent infection with M. tuberculosis or provide sterilizing immunity. While the understanding of the immune response generated against this pathogen is incomplete, improvements have been achieved due to advances in immunological tools. In this study, we analyzed the multifunctional nature of primary and memory CD8 T-cell responses generated during murine M. tuberculosis infection. We generated a recombinant M. tuberculosis strain expressing ovalbumin (OVA) epitopes in order to expand the peptides for the detection of CD8 T cells during M. tuberculosis infection and enable us to use OVA-specific reagents. Our results indicate that the majority of M. tuberculosis-specific CD8 T cells are limited to either cytotoxicity or the secretion of gamma interferon (IFN-gamma), with cytotoxicity being far more prevalent than IFN-gamma secretion. Memory CD8 T cells responded earlier and reached higher levels in the lungs than naïve CD8 T cells, as was expected. They were, however, less cytotoxic and secreted less IFN-gamma than newly primed CD8 T cells, suggesting that one factor contributing to bacterial persistence and lack of sterilizing immunity may be the low quality of memory cells that are generated.
Figures




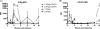
References
-
- Algood, H. M. S., P. L. Lin, D. Yankura, A. Jones, J. Chan, and J. L. Flynn. 2004. TNF influences chemokine expression of macrophages in vitro and that of CD11b+ cells in vivo during Mycobacterium tuberculosis infection. J. Immunol. 172:6846-6857. - PubMed
-
- Barber, D. L., E. J. Wherry, D. Masopust, B. Zhu, J. P. Allison, A. H. Sharpe, G. J. Freeman, and R. Ahmed. 2006. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439:682-687. - PubMed
-
- Betts, M. R., J. M. Brenchley, D. A. Price, S. C. De Rosa, D. C. Douek, M. Roederer, and R. A. Koup. 2003. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J. Immunol. Methods 281:65-78. - PubMed
-
- Bibb, L. A., and G. F. Hatfull. 2002. Integration and excision of the Mycobacterium tuberculosis prophage-like element, phiRv1. Mol. Microbiol. 45:1515-1526. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

