Cancer cell-derived microparticles bearing P-selectin glycoprotein ligand 1 accelerate thrombus formation in vivo
- PMID: 19667060
- PMCID: PMC2737159
- DOI: 10.1084/jem.20082297
Cancer cell-derived microparticles bearing P-selectin glycoprotein ligand 1 accelerate thrombus formation in vivo
Abstract
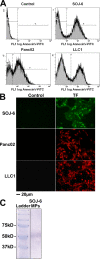
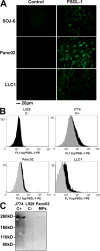
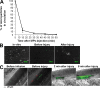
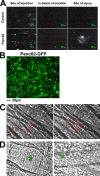
Recent publications have demonstrated the presence of tissue factor (TF)-bearing microparticles (MPs) in the blood of patients suffering from cancer. However, whether these MPs are involved in thrombosis remains unknown. We show that pancreatic and lung cancer cells produce MPs that express active TF and P-selectin glycoprotein ligand 1 (PSGL-1). Cancer cell-derived MPs aggregate platelets via a TF-dependent pathway. In vivo, cancer cell-derived MPs, but not their parent cells, infused into a living mouse accumulate at the site of injury and reduce tail bleeding time and the time to occlusion of venules and arterioles. This thrombotic state is also observed in mice developing tumors. In such mice, the amount of circulating platelet-, endothelial cell-, and cancer cell-derived MPs is increased. Endogenous cancer cell-derived MPs shed from the growing tumor are able to accumulate at the site of injury. Infusion of a blocking P-selectin antibody abolishes the thrombotic state observed after injection of MPs or in mice developing a tumor. Collectively, our results indicate that cancer cell-derived MPs bearing PSGL-1 and TF play a key role in thrombus formation in vivo. Targeting these MPs could be of clinical interest in the prevention of thrombosis and to limit formation of metastasis in cancer patients.
Figures



Similar articles
-
Tissue factor expressed by circulating cancer cell-derived microparticles drastically increases the incidence of deep vein thrombosis in mice.J Thromb Haemost. 2015 Jul;13(7):1310-9. doi: 10.1111/jth.13002. Epub 2015 Jun 8. J Thromb Haemost. 2015. PMID: 25955268 Free PMC article.
-
Activated Platelet-Derived and Leukocyte-Derived Circulating Microparticles and the Risk of Thrombosis in Heparin-Induced Thrombocytopenia: A Role for PF4-Bearing Microparticles?Cytometry B Clin Cytom. 2018 Mar;94(2):334-341. doi: 10.1002/cyto.b.21507. Epub 2017 Jan 13. Cytometry B Clin Cytom. 2018. PMID: 28052584
-
Inhibition of platelet activation prevents the P-selectin and integrin-dependent accumulation of cancer cell microparticles and reduces tumor growth and metastasis in vivo.Int J Cancer. 2015 Jan 15;136(2):462-75. doi: 10.1002/ijc.28997. Epub 2014 Jun 9. Int J Cancer. 2015. PMID: 24889539
-
Platelet microparticles and vascular cells interactions: a checkpoint between the haemostatic and thrombotic responses.Platelets. 2008 Feb;19(1):9-23. doi: 10.1080/09537100701817232. Platelets. 2008. PMID: 18231934 Review.
-
Tumor-derived tissue factor-positive microparticles and venous thrombosis in cancer patients.Blood. 2013 Sep 12;122(11):1873-80. doi: 10.1182/blood-2013-04-460139. Epub 2013 Jun 24. Blood. 2013. PMID: 23798713 Free PMC article. Review.
Cited by
-
Huisheng Oral Solution exerts anti-tumor effects by downregulating tissue factor and inhibiting the expression of metastasis-related factors, CD44, MMP2, and VEGF.Transl Cancer Res. 2019 Nov;8(7):2602-2612. doi: 10.21037/tcr.2019.10.25. Transl Cancer Res. 2019. PMID: 35117017 Free PMC article.
-
Managing thrombosis in cancer patients.Res Pract Thromb Haemost. 2018 May 1;2(3):429-438. doi: 10.1002/rth2.12102. eCollection 2018 Jul. Res Pract Thromb Haemost. 2018. PMID: 30046747 Free PMC article. Review.
-
Effects of the interactions between platelets with other cells in tumor growth and progression.Front Immunol. 2023 Apr 17;14:1165989. doi: 10.3389/fimmu.2023.1165989. eCollection 2023. Front Immunol. 2023. PMID: 37153586 Free PMC article. Review.
-
Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis.Cancer Cell. 2016 Dec 12;30(6):836-848. doi: 10.1016/j.ccell.2016.10.009. Cancer Cell. 2016. PMID: 27960084 Free PMC article. Review.
-
Differential contributions of monocyte- and platelet-derived microparticles towards thrombin generation and fibrin formation and stability.J Thromb Haemost. 2011 Nov;9(11):2251-61. doi: 10.1111/j.1538-7836.2011.04488.x. J Thromb Haemost. 2011. PMID: 21883880 Free PMC article.
References
-
- Abid Hussein M.N., Meesters E.W., Osmanovic N., Romijn F.P., Nieuwland R., Sturk A. 2003. Antigenic characterization of endothelial cell-derived microparticles and their detection ex vivo.J. Thromb. Haemost. 1:2434–2443 - PubMed
-
- Berckmans R.J., Neiuwland R., Böing A.N., Romijn F.P., Hack C.E., Sturk A. 2001. Cell-derived microparticles circulate in healthy humans and support low grade thrombin generation.Thromb. Haemost. 85:639–646 - PubMed
-
- Bertram J.S., Janik P. 1980. Establishment of a cloned line of Lewis Lung Carcinoma cells adapted to cell culture.Cancer Lett. 11:63–73 - PubMed
-
- Blom J.W., Osanto S., Rosendaal F.R. 2006a. High risk of venous thrombosis in patients with pancreatic cancer: a cohort study of 202 patients.Eur. J. Cancer. 42:410–414 - PubMed
-
- Blom J.W., Vanderschoot J.P., Oostindiër M.J., Osanto S., van der Meer F.J., Rosendaal F.R. 2006b. Incidence of venous thrombosis in a large cohort of 66,329 cancer patients: results of a record linkage study.J. Thromb. Haemost. 4:529–535 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

