Decreased TNF-alpha synthesis by macrophages restricts cutaneous immunosurveillance by memory CD4+ T cells during aging
- PMID: 19667063
- PMCID: PMC2737169
- DOI: 10.1084/jem.20090896
Decreased TNF-alpha synthesis by macrophages restricts cutaneous immunosurveillance by memory CD4+ T cells during aging
Abstract
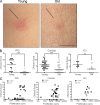
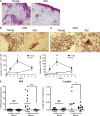
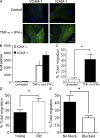
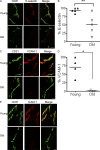
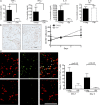
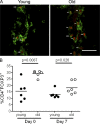
Immunity declines during aging, however the mechanisms involved in this decline are not known. In this study, we show that cutaneous delayed type hypersensitivity (DTH) responses to recall antigens are significantly decreased in older individuals. However, this is not related to CC chemokine receptor 4, cutaneous lymphocyte-associated antigen, or CD11a expression by CD4(+) T cells or their physical capacity for migration. Instead, there is defective activation of dermal blood vessels in older subject that results from decreased TNF-alpha secretion by macrophages. This prevents memory T cell entry into the skin after antigen challenge. However, isolated cutaneous macrophages from these subjects can be induced to secrete TNF-alpha after stimulation with Toll-like receptor (TLR) 1/2 or TLR 4 ligands in vitro, indicating that the defect is reversible. The decreased conditioning of tissue microenvironments by macrophage-derived cytokines may therefore lead to defective immunosurveillance by memory T cells. This may be a predisposing factor for the development of malignancy and infection in the skin during aging.
Figures


References
-
- Adamson P., Etienne S., Couraud P.O., Calder V., Greenwood J. 1999. Lymphocyte migration through brain endothelial cell monolayers involves signaling through endothelial ICAM-1 via a rho-dependent pathway.J. Immunol. 162:2964–2973 - PubMed
-
- Balato A., Balato N., Di Costanzo L., Ayala F. 2008. Contact sensitization of older patients in an academic department in Naples, Italy.Dermatitis. 19:209–212 - PubMed
-
- Berlin C., Bargatze R.F., Campbell J.J., von Andrian U.H., Szabo M.C., Hasslen S.R., Nelson R.D., Berg E.L., Erlandsen S.L., Butcher E.C. 1995. alpha 4 integrins mediate lymphocyte attachment and rolling under physiologic flow.Cell. 80:413–422 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

