Cell cycle-dependent role of MRN at dysfunctional telomeres: ATM signaling-dependent induction of nonhomologous end joining (NHEJ) in G1 and resection-mediated inhibition of NHEJ in G2
- PMID: 19667071
- PMCID: PMC2756883
- DOI: 10.1128/MCB.00476-09
Cell cycle-dependent role of MRN at dysfunctional telomeres: ATM signaling-dependent induction of nonhomologous end joining (NHEJ) in G1 and resection-mediated inhibition of NHEJ in G2
Abstract
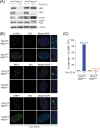
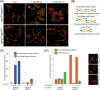
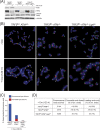
Here, we address the role of the MRN (Mre11/Rad50/Nbs1) complex in the response to telomeres rendered dysfunctional by deletion of the shelterin component TRF2. Using conditional NBS1/TRF2 double-knockout MEFs, we show that MRN is required for ATM signaling in response to telomere dysfunction. This establishes that MRN is the only sensor for the ATM kinase and suggests that TRF2 might block ATM signaling by interfering with MRN binding to the telomere terminus, possibly by sequestering the telomere end in the t-loop structure. We also examined the role of the MRN/ATM pathway in nonhomologous end joining (NHEJ) of damaged telomeres. NBS1 deficiency abrogated the telomere fusions that occur in G(1), consistent with the requirement for ATM and its target 53BP1 in this setting. Interestingly, NBS1 and ATM, but not H2AX, repressed NHEJ at dysfunctional telomeres in G(2), specifically at telomeres generated by leading-strand DNA synthesis. Leading-strand telomere ends were not prone to fuse in the absence of either TRF2 or MRN/ATM, indicating redundancy in their protection. We propose that MRN represses NHEJ by promoting the generation of a 3' overhang after completion of leading-strand DNA synthesis. TRF2 may ensure overhang formation by recruiting MRN (and other nucleases) to newly generated telomere ends. The activation of the MRN/ATM pathway by the dysfunctional telomeres is proposed to induce resection that protects the leading-strand ends from NHEJ when TRF2 is absent. Thus, the role of MRN at dysfunctional telomeres is multifaceted, involving both repression of NHEJ in G(2) through end resection and induction of NHEJ in G(1) through ATM-dependent signaling.
Figures


Similar articles
-
Multiple roles for MRE11 at uncapped telomeres.Nature. 2009 Aug 13;460(7257):914-8. doi: 10.1038/nature08196. Epub 2009 Jul 26. Nature. 2009. PMID: 19633651 Free PMC article.
-
NBS1 Phosphorylation Status Dictates Repair Choice of Dysfunctional Telomeres.Mol Cell. 2017 Mar 2;65(5):801-817.e4. doi: 10.1016/j.molcel.2017.01.016. Epub 2017 Feb 16. Mol Cell. 2017. PMID: 28216226 Free PMC article.
-
DNA processing is not required for ATM-mediated telomere damage response after TRF2 deletion.Nat Cell Biol. 2005 Jul;7(7):712-8. doi: 10.1038/ncb1275. Epub 2005 Jun 19. Nat Cell Biol. 2005. PMID: 15968270
-
Mre11-Rad50-Nbs1 is a keystone complex connecting DNA repair machinery, double-strand break signaling, and the chromatin template.Biochem Cell Biol. 2007 Aug;85(4):509-20. doi: 10.1139/O07-069. Biochem Cell Biol. 2007. PMID: 17713585 Review.
-
Mystery of DNA repair: the role of the MRN complex and ATM kinase in DNA damage repair.J Appl Genet. 2008;49(4):383-96. doi: 10.1007/BF03195638. J Appl Genet. 2008. PMID: 19029686 Review.
Cited by
-
High-throughput screen to identify compounds that prevent or target telomere loss in human cancer cells.NAR Cancer. 2022 Oct 3;4(4):zcac029. doi: 10.1093/narcan/zcac029. eCollection 2022 Dec. NAR Cancer. 2022. PMID: 36196242 Free PMC article.
-
Lymphoid-specific helicase in epigenetics, DNA repair and cancer.Br J Cancer. 2022 Feb;126(2):165-173. doi: 10.1038/s41416-021-01543-2. Epub 2021 Sep 7. Br J Cancer. 2022. PMID: 34493821 Free PMC article. Review.
-
DNA repair at telomeres: keeping the ends intact.Cold Spring Harb Perspect Biol. 2013 Jun 1;5(6):a012666. doi: 10.1101/cshperspect.a012666. Cold Spring Harb Perspect Biol. 2013. PMID: 23732473 Free PMC article. Review.
-
SNMIB/Apollo protects leading-strand telomeres against NHEJ-mediated repair.EMBO J. 2010 Jul 7;29(13):2230-41. doi: 10.1038/emboj.2010.58. Epub 2010 Jun 15. EMBO J. 2010. PMID: 20551906 Free PMC article.
-
MRN and the race to the break.Chromosoma. 2010 Apr;119(2):115-35. doi: 10.1007/s00412-009-0242-4. Epub 2009 Oct 28. Chromosoma. 2010. PMID: 19862546 Review.
References
-
- Bai, Y., and J. P. Murnane. 2003. Telomere instability in a human tumor cell line expressing NBS1 with mutations at sites phosphorylated by ATM. Mol. Cancer Res. 1:1058-1069. - PubMed
-
- Bailey, S. M., M. N. Cornforth, A. Kurimasa, D. J. Chen, and E. H. Goodwin. 2001. Strand-specific postreplicative processing of mammalian telomeres. Science 293:2462-2465. - PubMed
-
- Bailey, S. M., E. H. Goodwin, and M. N. Cornforth. 2004. Strand-specific fluorescence in situ hybridization: the CO-FISH family. Cytogenet. Genome Res. 107:14-17. - PubMed
-
- Bailey, S. M., M. A. Brenneman, J. Halbrook, J. A. Nickoloff, R. L. Ullrich, and E. H. Goodwin. 2004. The kinase activity of DNA-PK is required to protect mammalian telomeres. DNA Repair (Amsterdam) 3:225-233. - PubMed
-
- Bassing, C. H., K. F. Chua, J. Sekiguchi, H. Suh, S. R. Whitlow, J. C. Fleming, B. C. Monroe, D. N. Ciccone, C. Yan, K. Vlasakova, D. M. Livingston, D. O. Ferguson, R. Scully, and F. W. Alt. 2002. Increased ionizing radiation sensitivity and genomic instability in the absence of histone H2AX. Proc. Natl. Acad. Sci. USA 99:8173-8178. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
