Modulation of Rho guanine exchange factor Lfc activity by protein kinase A-mediated phosphorylation
- PMID: 19667072
- PMCID: PMC2772734
- DOI: 10.1128/MCB.01268-08
Modulation of Rho guanine exchange factor Lfc activity by protein kinase A-mediated phosphorylation
Abstract
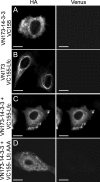
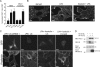
Lfc is a guanine nucleotide exchange factor (GEF) for Rho that demonstrates an unusual ability to associate with microtubules. While several phosphorylated residues have been detected in the Lfc polypeptide, the mechanism(s) by which phosphorylation regulates the exchange activity of Lfc remains unclear. We confirm that Lfc is a phosphorylated protein and demonstrate that 14-3-3 interacts directly and in a phosphorylation-dependent manner with Lfc. We identify AKAP121 as an Lfc-binding protein and show that Lfc is phosphorylated in an AKAP-dependent manner by protein kinase A (PKA). Forskolin treatment induced 14-3-3 binding to Lfc and suppressed the exchange activity of wild-type Lfc on RhoA. Importantly, a mutant of Lfc that is unable to associate with 14-3-3 proteins was resistant to inhibition by forskolin. Tctex-1, a dynein motor light chain, binds to Lfc in a competitive manner with 14-3-3.
Figures




Similar articles
-
Mechanistic insight into the microtubule and actin cytoskeleton coupling through dynein-dependent RhoGEF inhibition.Mol Cell. 2012 Mar 9;45(5):642-55. doi: 10.1016/j.molcel.2012.01.027. Mol Cell. 2012. PMID: 22405273
-
Involvement of p114-RhoGEF and Lfc in Wnt-3a- and dishevelled-induced RhoA activation and neurite retraction in N1E-115 mouse neuroblastoma cells.Mol Biol Cell. 2010 Oct 15;21(20):3590-600. doi: 10.1091/mbc.E10-02-0095. Epub 2010 Sep 1. Mol Biol Cell. 2010. PMID: 20810787 Free PMC article.
-
Mechanistic insight into GPCR-mediated activation of the microtubule-associated RhoA exchange factor GEF-H1.Nat Commun. 2014 Sep 11;5:4857. doi: 10.1038/ncomms5857. Nat Commun. 2014. PMID: 25209408
-
A novel mechanism of G protein-dependent phosphorylation of vasodilator-stimulated phosphoprotein.J Biol Chem. 2005 Sep 23;280(38):32866-76. doi: 10.1074/jbc.M501361200. Epub 2005 Jul 26. J Biol Chem. 2005. PMID: 16046415
-
AKAP-Lbc: a molecular scaffold for the integration of cyclic AMP and Rho transduction pathways.Eur J Cell Biol. 2006 Jul;85(7):603-10. doi: 10.1016/j.ejcb.2006.01.001. Epub 2006 Feb 7. Eur J Cell Biol. 2006. PMID: 16460837 Review.
Cited by
-
GEF-H1 controls microtubule-dependent sensing of nucleic acids for antiviral host defenses.Nat Immunol. 2014 Jan;15(1):63-71. doi: 10.1038/ni.2766. Epub 2013 Nov 24. Nat Immunol. 2014. PMID: 24270516 Free PMC article.
-
Evidence for the involvement of Lfc and Tctex-1 in axon formation.J Neurosci. 2010 May 12;30(19):6793-800. doi: 10.1523/JNEUROSCI.5420-09.2010. J Neurosci. 2010. PMID: 20463241 Free PMC article.
-
Tyrosine Phosphorylation of SGEF Regulates RhoG Activity and Cell Migration.PLoS One. 2016 Jul 20;11(7):e0159617. doi: 10.1371/journal.pone.0159617. eCollection 2016. PLoS One. 2016. PMID: 27437949 Free PMC article.
-
14-3-3 proteins regulate protein kinase a activity to modulate growth cone turning responses.J Neurosci. 2010 Oct 20;30(42):14059-67. doi: 10.1523/JNEUROSCI.3883-10.2010. J Neurosci. 2010. PMID: 20962227 Free PMC article.
-
The role of microtubules in the regulation of epithelial junctions.Tissue Barriers. 2018;6(3):1539596. doi: 10.1080/21688370.2018.1539596. Epub 2018 Nov 5. Tissue Barriers. 2018. PMID: 30395792 Free PMC article. Review.
References
-
- Aghazadeh, B., W. E. Lowry, X. Y. Huang, and M. K. Rosen. 2000. Structural basis for relief of autoinhibition of the Dbl homology domain of proto-oncogene Vav by tyrosine phosphorylation. Cell 102:625-633. - PubMed
-
- Aijaz, S., F. D'Atri, S. Citi, M. S. Balda, and K. Matter. 2005. Binding of GEF-H1 to the tight junction-associated adaptor cingulin results in inhibition of Rho signaling and G1/S phase transition. Dev. Cell 8:777-786. - PubMed
-
- Alam, M. R., R. C. Johnson, D. N. Darlington, T. A. Hand, R. E. Mains, and B. A. Eipper. 1997. Kalirin, a cytosolic protein with spectrin-like and GDP/GTP exchange factor-like domains that interacts with peptidylglycine alpha-amidating monooxygenase, an integral membrane peptide-processing enzyme. J. Biol. Chem. 272:12667-12675. - PubMed
-
- Baisamy, L., N. Jurisch, and D. Diviani. 2005. Leucine zipper-mediated homo-oligomerization regulates the Rho-GEF activity of AKAP-Lbc. J. Biol. Chem. 280:15405-15412. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
