Modulation of Rho guanine exchange factor Lfc activity by protein kinase A-mediated phosphorylation
- PMID: 19667072
- PMCID: PMC2772734
- DOI: 10.1128/MCB.01268-08
Modulation of Rho guanine exchange factor Lfc activity by protein kinase A-mediated phosphorylation
Abstract
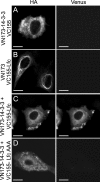
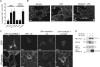
Lfc is a guanine nucleotide exchange factor (GEF) for Rho that demonstrates an unusual ability to associate with microtubules. While several phosphorylated residues have been detected in the Lfc polypeptide, the mechanism(s) by which phosphorylation regulates the exchange activity of Lfc remains unclear. We confirm that Lfc is a phosphorylated protein and demonstrate that 14-3-3 interacts directly and in a phosphorylation-dependent manner with Lfc. We identify AKAP121 as an Lfc-binding protein and show that Lfc is phosphorylated in an AKAP-dependent manner by protein kinase A (PKA). Forskolin treatment induced 14-3-3 binding to Lfc and suppressed the exchange activity of wild-type Lfc on RhoA. Importantly, a mutant of Lfc that is unable to associate with 14-3-3 proteins was resistant to inhibition by forskolin. Tctex-1, a dynein motor light chain, binds to Lfc in a competitive manner with 14-3-3.
Figures




References
-
- Aghazadeh, B., W. E. Lowry, X. Y. Huang, and M. K. Rosen. 2000. Structural basis for relief of autoinhibition of the Dbl homology domain of proto-oncogene Vav by tyrosine phosphorylation. Cell 102:625-633. - PubMed
-
- Aijaz, S., F. D'Atri, S. Citi, M. S. Balda, and K. Matter. 2005. Binding of GEF-H1 to the tight junction-associated adaptor cingulin results in inhibition of Rho signaling and G1/S phase transition. Dev. Cell 8:777-786. - PubMed
-
- Alam, M. R., R. C. Johnson, D. N. Darlington, T. A. Hand, R. E. Mains, and B. A. Eipper. 1997. Kalirin, a cytosolic protein with spectrin-like and GDP/GTP exchange factor-like domains that interacts with peptidylglycine alpha-amidating monooxygenase, an integral membrane peptide-processing enzyme. J. Biol. Chem. 272:12667-12675. - PubMed
-
- Baisamy, L., N. Jurisch, and D. Diviani. 2005. Leucine zipper-mediated homo-oligomerization regulates the Rho-GEF activity of AKAP-Lbc. J. Biol. Chem. 280:15405-15412. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
