The adapter protein SLP-76 mediates "outside-in" integrin signaling and function in T cells
- PMID: 19667077
- PMCID: PMC2756887
- DOI: 10.1128/MCB.00283-09
The adapter protein SLP-76 mediates "outside-in" integrin signaling and function in T cells
Abstract
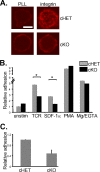
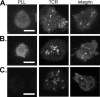
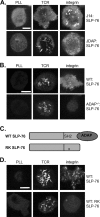
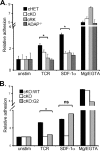
The adapter protein SH2 domain-containing leukocyte protein of 76 kDa (SLP-76) is an essential mediator of signaling from the T-cell antigen receptor (TCR). We report here that SLP-76 also mediates signaling downstream of integrins in T cells and that SLP-76-deficient T cells fail to support adhesion to integrin ligands. In response to both TCR and integrin stimulation, SLP-76 relocalizes to surface microclusters that colocalize with phosphorylated signaling proteins. Disruption of SLP-76 recruitment to the protein named LAT (linker for activation of T cells) inhibits SLP-76 clustering downstream of the TCR but not downstream of integrins. Conversely, an SLP-76 mutant unable to bind ADAP (adhesion and degranulation-promoting adapter protein) forms clusters following TCR but not integrin engagement and fails to support T-cell adhesion to integrin ligands. These findings demonstrate that SLP-76 relocalizes to integrin-initiated signaling complexes by a mechanism different from that employed during TCR signaling and that SLP-76 relocalization corresponds to SLP-76-dependent integrin function in T cells.
Figures




References
-
- Abraham, R. T., and A. Weiss. 2004. Jurkat T cells and development of the T-cell receptor signalling paradigm. Nat. Rev. Immunol. 4:301-308. - PubMed
-
- Baker, R. G., and G. A. Koretzky. 2008. Regulation of T cell integrin function by adapter proteins. Immunol. Res. 42:132-144. - PubMed
-
- Barr, V. A., L. Balagopalan, M. Barda-Saad, R. Polishchuk, H. Boukari, S. C. Bunnell, K. M. Bernot, Y. Toda, R. Nossal, and L. E. Samelson. 2006. T-cell antigen receptor-induced signaling complexes: internalization via a cholesterol-dependent endocytic pathway. Traffic 7:1143-1162. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
