Asymmetry of inner dynein arms and inter-doublet links in Chlamydomonas flagella
- PMID: 19667131
- PMCID: PMC2728406
- DOI: 10.1083/jcb.200903082
Asymmetry of inner dynein arms and inter-doublet links in Chlamydomonas flagella
Abstract
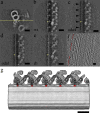
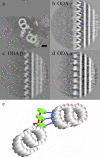
Although the widely shared "9 + 2" structure of axonemes is thought to be highly symmetrical, axonemes show asymmetrical bending during planar and conical motion. In this study, using electron cryotomography and single particle averaging, we demonstrate an asymmetrical molecular arrangement of proteins binding to the nine microtubule doublets in Chlamydomonas reinhardtii flagella. The eight inner arm dynein heavy chains regulate and determine flagellar waveform. Among these, one heavy chain (dynein c) is missing on one microtubule doublet (this doublet also lacks the outer dynein arm), and another dynein heavy chain (dynein b or g) is missing on the adjacent doublet. Some dynein heavy chains either show an abnormal conformation or were replaced by other proteins, possibly minor dyneins. In addition to nexin, there are two additional linkages between specific pairs of doublets. Interestingly, all these exceptional arrangements take place on doublets on opposite sides of the axoneme, suggesting that the transverse functional asymmetry of the axoneme causes an in-plane bending motion.
Figures



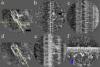
References
-
- Bannai H., Yoshimura M., Takahashi K., Shingyoji C. 2000. Calcium regulation of microtubule sliding in reactivated sea urchin sperm flagella.J. Cell Sci. 113:831–839 - PubMed
-
- Bozkurt H.H., Woolley D.M. 1993. Morphology of nexin links in relation to interdoublet sliding in the sperm flagellum.Cell Motil. Cytoskeleton. 24:109–118 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

