Highly pathogenic H5N1 influenza virus can enter the central nervous system and induce neuroinflammation and neurodegeneration
- PMID: 19667183
- PMCID: PMC2729020
- DOI: 10.1073/pnas.0900096106
Highly pathogenic H5N1 influenza virus can enter the central nervous system and induce neuroinflammation and neurodegeneration
Abstract
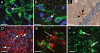
One of the greatest influenza pandemic threats at this time is posed by the highly pathogenic H5N1 avian influenza viruses. To date, 61% of the 433 known human cases of H5N1 infection have proved fatal. Animals infected by H5N1 viruses have demonstrated acute neurological signs ranging from mild encephalitis to motor disturbances to coma. However, no studies have examined the longer-term neurologic consequences of H5N1 infection among surviving hosts. Using the C57BL/6J mouse, a mouse strain that can be infected by the A/Vietnam/1203/04 H5N1 virus without adaptation, we show that this virus travels from the peripheral nervous system into the CNS to higher levels of the neuroaxis. In regions infected by H5N1 virus, we observe activation of microglia and alpha-synuclein phosphorylation and aggregation that persists long after resolution of the infection. We also observe a significant loss of dopaminergic neurons in the substantia nigra pars compacta 60 days after infection. Our results suggest that a pandemic H5N1 pathogen, or other neurotropic influenza virus, could initiate CNS disorders of protein aggregation including Parkinson's and Alzheimer's diseases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- World Health Organization. Cumulative Number of Confirmed Human Cases of Avian Influenza A/(H5N1) Reported to WHO. Geneva: WHO; 2008.
-
- Maurizi CP. Why was the 1918 influenza pandemic so lethal? The possible role of a neurovirulent neuraminidase. Med Hypotheses. 1985;16:1–5. - PubMed
-
- Ravenholt RT, Foege WH. 1918 influenza, encephalitis lethargica, parkinsonism. Lancet. 1982;2:860–864. - PubMed
-
- Tanimura N, et al. Pathology of fatal highly pathogenic H5N1 avian influenza virus infection in large-billed crows (Corvus macrorhynchos) during the 2004 outbreak in Japan. Vet Pathol. 2006;43:500–509. - PubMed
-
- de Jong MD, et al. Fatal avian influenza A (H5N1) in a child presenting with diarrhea followed by coma. N Engl J Med. 2005;352:686–691. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

