A role for the TGFbeta-Par6 polarity pathway in breast cancer progression
- PMID: 19667198
- PMCID: PMC2729014
- DOI: 10.1073/pnas.0906796106
A role for the TGFbeta-Par6 polarity pathway in breast cancer progression
Abstract
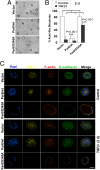
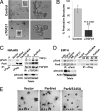
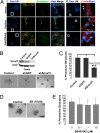
The role of polarity signaling in cancer metastasis is ill defined. Using two three-dimensional culture models of mammary epithelial cells and an orthotopic mouse model of breast cancer, we reveal that Par6 signaling, which is regulated directly by TGFbeta, plays a role in breast cancer metastasis. Interference with Par6 signaling blocked TGFbeta-dependent loss of polarity in acini-like structures formed by non-transformed mammary cells grown in three-dimensional structures and suppressed the protrusive morphology of mesenchymal-like invasive mammary tumor cells without rescuing E-cadherin expression. Moreover, blockade of Par6 signaling in an in vivo orthotopic model of metastatic breast cancer induced the formation of ZO-1-positive epithelium-like structures in the primary tumor and suppressed metastasis to the lungs. Analysis of the pathway in tissue microarrays of human breast tumors further revealed that Par6 activation correlated with markers of the basal carcinoma subtype in BRCA1-associated tumors. These studies thus reveal a key role for polarity signaling and the control of morphologic transformation in breast cancer metastasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Padua D, Massague J. Roles of TGFbeta in metastasis. Cell Res. 2009;19:89–102. - PubMed
-
- Zavadil J, Bottinger EP. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene. 2005;24:5764–5774. - PubMed
-
- Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17:548–558. - PubMed
-
- Bose R, Wrana JL. Regulation of Par6 by extracellular signals. Curr Opin Cell Biol. 2006;18:206–212. - PubMed
-
- Ozdamar B, et al. Regulation of the polarity protein Par6 by TGFbeta receptors controls epithelial cell plasticity. Science. 2005;307:1603–1609. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

