Evolutionary and functional insights into the mechanism underlying high-altitude adaptation of deer mouse hemoglobin
- PMID: 19667207
- PMCID: PMC2732835
- DOI: 10.1073/pnas.0905224106
Evolutionary and functional insights into the mechanism underlying high-altitude adaptation of deer mouse hemoglobin
Abstract
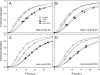
Adaptive modifications of heteromeric proteins may involve genetically based changes in single subunit polypeptides or parallel changes in multiple genes that encode distinct, interacting subunits. Here we investigate these possibilities by conducting a combined evolutionary and functional analysis of duplicated globin genes in natural populations of deer mice (Peromyscus maniculatus) that are adapted to different elevational zones. A multilocus analysis of nucleotide polymorphism and linkage disequilibrium revealed that high-altitude adaptation of deer mouse hemoglobin involves parallel functional differentiation at multiple unlinked gene duplicates: two alpha-globin paralogs on chromosome 8 and two beta-globin paralogs on chromosome 1. Differences in O(2)-binding affinity of the alternative beta-chain hemoglobin isoforms were entirely attributable to allelic differences in sensitivity to 2,3-diphosphoglycerate (DPG), an allosteric cofactor that stabilizes the low-affinity, deoxygenated conformation of the hemoglobin tetramer. The two-locus beta-globin haplotype that predominates at high altitude is associated with suppressed DPG-sensitivity (and hence, increased hemoglobin-O(2) affinity), which enhances pulmonary O(2) loading under hypoxia. The discovery that allelic differences in DPG-sensitivity contribute to adaptive variation in hemoglobin-O(2) affinity illustrates the value of integrating evolutionary analyses of sequence variation with mechanistic appraisals of protein function. Investigation into the functional significance of the deer mouse beta-globin polymorphism was motivated by the results of population genetic analyses which revealed evidence for a history of divergent selection between elevational zones. The experimental measures of O(2)-binding properties corroborated the tests of selection by demonstrating a functional difference between the products of alternative alleles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Perutz MF. Mechanisms of cooperativity and allosteric regulation in proteins. Q RevBiophys. 1989;22:139–236. - PubMed
-
- Perutz MF. Species adaptation in a protein molecule. Mol Biol Evol. 1983;1:1–28. - PubMed
-
- Weber RE, Fago A. Functional adaptation and its molecular basis in vertebrate hemoglobins, neuroglobins and cytoglobins. Respir Physiol Neurobiol. 2004;144:141–159. - PubMed
-
- Monge C, León-Velarde F. Physiological adaptation to high altitude: Oxygen transport in mammals and birds. Physiol Rev. 1991;71:1135–1172. - PubMed
-
- Storz JF. Hemoglobin function and physiological adaptation to hypoxia in high-altitude mammals. J Mammal. 2007;88:24–31.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

