Ca2+ regulation in the Na+/Ca2+ exchanger features a dual electrostatic switch mechanism
- PMID: 19667209
- PMCID: PMC2732890
- DOI: 10.1073/pnas.0902171106
Ca2+ regulation in the Na+/Ca2+ exchanger features a dual electrostatic switch mechanism
Abstract
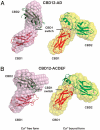
Regulation of ion-transport in the Na(+)/Ca(2+) exchanger (NCX) occurs via its cytoplasmic Ca(2+)-binding domains, CBD1 and CBD2. Here, we present a mechanism for NCX activation and inactivation based on data obtained using NMR, isothermal titration calorimetry (ITC) and small-angle X-ray scattering (SAXS). We initially determined the structure of the Ca(2+)-free form of CBD2-AD and the structure of CBD2-BD that represent the two major splice variant classes in NCX1. Although the apo-form of CBD2-AD displays partially disordered Ca(2+)-binding sites, those of CBD2-BD are entirely unstructured even in an excess of Ca(2+). Striking differences in the electrostatic potential between the Ca(2+)-bound and -free forms strongly suggest that Ca(2+)-binding sites in CBD1 and CBD2 form electrostatic switches analogous to C(2)-domains. SAXS analysis of a construct containing CBD1 and CBD2 reveals a conformational change mediated by Ca(2+)-binding to CBD1. We propose that the electrostatic switch in CBD1 and the associated conformational change are necessary for exchanger activation. The response of the CBD1 switch to intracellular Ca(2+) is influenced by the closely located cassette exons. We further propose that Ca(2+)-binding to CBD2 induces a second electrostatic switch, required to alleviate Na(+)-dependent inactivation of Na(+)/Ca(2+) exchange. In contrast to CBD1, the electrostatic switch in CBD2 is isoform- and splice variant-specific and allows for tailored exchange activities.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Ca(2+) regulation in the Na(+)/Ca (2+) exchanger features a dual electrostatic switch mechanism.Adv Exp Med Biol. 2013;961:27-33. doi: 10.1007/978-1-4614-4756-6_3. Adv Exp Med Biol. 2013. PMID: 23224867 Review.
-
Structure-dynamic determinants governing a mode of regulatory response and propagation of allosteric signal in splice variants of Na+/Ca2+ exchange (NCX) proteins.Biochem J. 2015 Feb 1;465(3):489-501. doi: 10.1042/BJ20141036. Biochem J. 2015. PMID: 25387769
-
Ca2+ regulation of ion transport in the Na+/Ca2+ exchanger.J Biol Chem. 2012 Sep 14;287(38):31641-9. doi: 10.1074/jbc.R112.353573. Epub 2012 Jul 20. J Biol Chem. 2012. PMID: 22822067 Free PMC article. Review.
-
Crystal structure of CBD2 from the Drosophila Na(+)/Ca(2+) exchanger: diversity of Ca(2+) regulation and its alternative splicing modification.J Mol Biol. 2009 Mar 20;387(1):104-12. doi: 10.1016/j.jmb.2009.01.045. Epub 2009 Jan 29. J Mol Biol. 2009. PMID: 19361442
-
Structure-Dynamic Coupling Through Ca2+-Binding Regulatory Domains of Mammalian NCX Isoform/Splice Variants.Adv Exp Med Biol. 2017;981:41-58. doi: 10.1007/978-3-319-55858-5_3. Adv Exp Med Biol. 2017. PMID: 29594857 Review.
Cited by
-
Development of a high-affinity peptide that prevents phospholemman (PLM) inhibition of the sodium/calcium exchanger 1 (NCX1).Biochem J. 2016 Aug 1;473(15):2413-23. doi: 10.1042/BCJ20160465. Epub 2016 May 31. Biochem J. 2016. PMID: 27247424 Free PMC article.
-
Electromechanical coupling in the cardiac myocyte; stretch-arrhythmia feedback.Pflugers Arch. 2011 Jul;462(1):165-75. doi: 10.1007/s00424-011-0944-3. Epub 2011 Mar 4. Pflugers Arch. 2011. PMID: 21373861 Review.
-
Structural dynamics of Na+ and Ca2+ interactions with full-size mammalian NCX.Commun Biol. 2024 Apr 16;7(1):463. doi: 10.1038/s42003-024-06159-9. Commun Biol. 2024. PMID: 38627576 Free PMC article.
-
Structural insight into the allosteric inhibition of human sodium-calcium exchanger NCX1 by XIP and SEA0400.EMBO J. 2024 Jan;43(1):14-31. doi: 10.1038/s44318-023-00013-0. Epub 2023 Dec 15. EMBO J. 2024. PMID: 38177313 Free PMC article.
-
Proton-sensing Ca2+ binding domains regulate the cardiac Na+/Ca2+ exchanger.J Biol Chem. 2011 Aug 19;286(33):28811-28820. doi: 10.1074/jbc.M110.214106. Epub 2011 Jun 16. J Biol Chem. 2011. PMID: 21680748 Free PMC article.
References
-
- Lytton J. Na+/Ca2+ exchangers: Three mammalian gene families control Ca2+ transport. Biochem J. 2007;406:365–382. - PubMed
-
- Kang TM, Hilgemann DW. Multiple transport modes of the cardiac Na+/Ca2+ exchanger. Nature. 2004;427:544–548. - PubMed
-
- Iwamoto T, et al. Unique topology of the internal repeats in the cardiac Na+/Ca2+ exchanger. FEBS Lett. 1999;446:264–268. - PubMed
-
- Nicoll DA, Ottolia M, Lu L, Lu Y, Philipson KD. A new topological model of the cardiac sarcolemmal Na+/Ca2+ exchanger. J Biol Chem. 1999;274:910–917. - PubMed
-
- Hilge M, Aelen J, Vuister GW. Ca2+ regulation in the Na+/Ca2+ exchanger involves two markedly different Ca2+ sensors. Mol Cell. 2006;22:15–25. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

