Prospective randomized trial of six-month versus nine-month therapy for intestinal tuberculosis
- PMID: 19667282
- PMCID: PMC2764179
- DOI: 10.1128/AAC.00874-09
Prospective randomized trial of six-month versus nine-month therapy for intestinal tuberculosis
Abstract
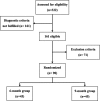
Intestinal tuberculosis (TB) continues to be a common disease worldwide. However, the optimal duration of anti-TB medication has not been well established. We therefore compared the efficacy of 6-month and 9-month therapy in the treatment of intestinal TB. Ninety patients definitely diagnosed with intestinal TB were randomized into 6-month (n = 45) or 9-month (n = 45) treatment groups, prospectively. The primary end point was complete response, defined as endoscopic healing of active lesions. Patients were followed up monthly for 3 months after therapy initiation, then every 3 months until the end of therapy, and finally 1 year later. Relapse was assessed 1 year after the end of therapy by patient interview and colonoscopy. Baseline characteristics were similar in the 6-month and 9-month groups. Intention-to-treat analysis revealed no significant differences between the two groups in complete response (6-month group, 93.3%; 9-month group, 91.1%; P = 1.00) or recurrence rate (6-month group, 2.4%; 9-month group, 0.0%; P = 1.00). Median follow-up duration was 39 months in the 6-month group and 32 months in the 9-month group. No surgery was performed on any patient in either group. In conclusion, the 6-month therapy was as effective as 9-month therapy in patients with intestinal TB and may have the additional benefits of reduced treatment cost and increased compliance.
References
-
- American Thoracic Society. 1992. Control of tuberculosis in the United States. Am. Rev. Respir. Dis. 146:1623-1633. - PubMed
-
- Bastani, B., M. R. Shariatzadeh, and F. Dehdashti. 1985. Tuberculous peritonitis—report of 30 cases and review of the literature. Q. J. Med. 56:549-557. - PubMed
-
- Blumberg, H. M., W. J. Burman, R. E. Chaisson, C. L. Daley, S. C. Etkind, L. N. Friedman, P. Fujiwara, M. Grzemska, P. C. Hopewell, M. D. Iseman, R. M. Jasmer, V. Koppaka, R. I. Menzies, R. J. O'Brien, R. R. Reves, L. B. Reichman, P. M. Simone, J. R. Starke, A. A. Vernon, et al. 2003. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am. J. Respir. Crit. Care Med. 167:603-662. - PubMed
-
- British Thoracic Association. 1982. A controlled trial of six months chemotherapy in pulmonary tuberculosis. Second report: results during the 24 months after the end of chemotherapy. Am. Rev. Respir. Dis. 126:460-462. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources