A novel role for c-Src and STAT3 in apoptotic cell-mediated MerTK-dependent immunoregulation of dendritic cells
- PMID: 19667404
- PMCID: PMC2759647
- DOI: 10.1182/blood-2009-03-207522
A novel role for c-Src and STAT3 in apoptotic cell-mediated MerTK-dependent immunoregulation of dendritic cells
Abstract
Dendritic cells (DCs) play an instrumental role in regulating tolerance to self-antigens and preventing autoimmunity. One mechanism by which "tolerogenic" DCs are established is through the inhibitory effects of apoptotic cells (ACs). Immature DCs encountering ACs are resistant to stimuli that activate and mature DCs. We have shown that the Mer receptor tyrosine kinase (MerTK) plays a key role in transducing inhibitory signals upon binding of ACs, which in turn involve the phosphatidylinositol 3-kinase (PI3K) pathway. Nevertheless, the molecular basis for AC-induced inhibition of DCs is ill defined. In the current study, the proximal signaling events induced by MerTK after AC binding were studied. AC treatment of bone marrow-derived or splenic DCs established a complex consisting of MerTK, the nonreceptor tyrosine kinase c-Src, the transcription factor STAT3, and PI3K. In contrast, AC treatment of DCs lacking MerTK expression failed to increase c-Src and STAT3 activation. In addition, the inhibitory effects of ACs were blocked by treating DCs with pharmacologic inhibitors or siRNA specific for c-Src and STAT3. These findings demonstrate that AC-induced inhibition of DCs requires MerTK-dependent activation of c-Src and STAT3, and provide evidence for novel roles for c-Src and STAT3 in the immunoregulation of DCs.
Figures
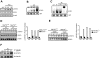
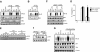
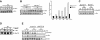
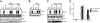
Similar articles
-
Apoptotic cells induce Mer tyrosine kinase-dependent blockade of NF-kappaB activation in dendritic cells.Blood. 2007 Jan 15;109(2):653-60. doi: 10.1182/blood-2006-04-017368. Epub 2006 Sep 28. Blood. 2007. PMID: 17008547 Free PMC article.
-
MerTK is required for apoptotic cell-induced T cell tolerance.J Exp Med. 2008 Jan 21;205(1):219-32. doi: 10.1084/jem.20062293. Epub 2008 Jan 14. J Exp Med. 2008. PMID: 18195070 Free PMC article.
-
MERTK interactions with SH2-domain proteins in the retinal pigment epithelium.PLoS One. 2013;8(2):e53964. doi: 10.1371/journal.pone.0053964. Epub 2013 Feb 4. PLoS One. 2013. PMID: 23390493 Free PMC article.
-
Molecular pathways: MERTK signaling in cancer.Clin Cancer Res. 2013 Oct 1;19(19):5275-80. doi: 10.1158/1078-0432.CCR-12-1451. Epub 2013 Jul 5. Clin Cancer Res. 2013. PMID: 23833304 Free PMC article. Review.
-
Recent advancements in role of TAM receptors on efferocytosis, viral infection, autoimmunity, and tissue repair.Int Rev Cell Mol Biol. 2020;357:1-19. doi: 10.1016/bs.ircmb.2020.09.008. Epub 2020 Nov 7. Int Rev Cell Mol Biol. 2020. PMID: 33234241 Review.
Cited by
-
Blocking elevated p38 MAPK restores efferocytosis and inflammatory resolution in the elderly.Nat Immunol. 2020 Jun;21(6):615-625. doi: 10.1038/s41590-020-0646-0. Epub 2020 Apr 6. Nat Immunol. 2020. PMID: 32251403 Free PMC article.
-
Mechanism of Mer receptor tyrosine kinase inhibition of glomerular endothelial cell inflammation.J Leukoc Biol. 2018 Apr;103(4):709-717. doi: 10.1002/JLB.3A0917-368R. Epub 2018 Jan 19. J Leukoc Biol. 2018. PMID: 29350876 Free PMC article.
-
Autologous apoptotic cells preceding transplantation enhance survival in lethal murine graft-versus-host models.Blood. 2014 Sep 11;124(11):1832-42. doi: 10.1182/blood-2014-02-555128. Epub 2014 Jul 16. Blood. 2014. PMID: 25030062 Free PMC article.
-
ACK1 and BRK non-receptor tyrosine kinase deficiencies are associated with familial systemic lupus and involved in efferocytosis.medRxiv [Preprint]. 2024 Jun 5:2024.02.15.24302255. doi: 10.1101/2024.02.15.24302255. medRxiv. 2024. Update in: Elife. 2024 Nov 21;13:RP96085. doi: 10.7554/eLife.96085. PMID: 38883731 Free PMC article. Updated. Preprint.
-
Molecular control of steady-state dendritic cell maturation and immune homeostasis.Annu Rev Immunol. 2013;31:743-91. doi: 10.1146/annurev-immunol-020711-074929. Epub 2013 Jan 17. Annu Rev Immunol. 2013. PMID: 23330953 Free PMC article. Review.
References
-
- Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. - PubMed
-
- Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. - PubMed
-
- Stuart LM, Lucas M, Simpson C, Lamb J, Savill J, Lacy-Hulbert A. Inhibitory effects of apoptotic cell ingestion upon endotoxin-driven myeloid dendritic cell maturation. J Immunol. 2002;168(4):1627–1635. - PubMed
-
- Gaipl US, Franz S, Voll RE, Sheriff A, Kalden JR, Herrmann M. Defects in the disposal of dying cells lead to autoimmunity. Curr Rheumatol. 2004;6(6):401–407. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

