Development and validation of the high-quality 'rapid method for swab' to genotype the HTTLPR serotonin transporter (SLC6A4) promoter polymorphism
- PMID: 19668112
- PMCID: PMC2730883
- DOI: 10.1097/YPG.0b013e3283208091
Development and validation of the high-quality 'rapid method for swab' to genotype the HTTLPR serotonin transporter (SLC6A4) promoter polymorphism
Abstract
Background: The importance of genetic variation to the etiology of neuropsychiatric disorders is well established and is currently being examined for diagnosis and treatment. The most popular method of obtaining material for genotype analysis, high-yielding DNA extraction from blood, has several limitations, including invasiveness, need for skilled individuals to collect material, and requirement for cold storage. Saliva sampling is noninvasive and trained personnel are less necessary, but it still requires a relatively high level of subject compliance. Buccal mucosa cells sampling is almost completely noninvasive, reducing compliance issues significantly. Samples collected have been shown to produce usable DNA after shipment through conventional mail. The DNA produced by rapid elution of these swabs in chaotropic buffers is, however, of limited quality and low purity.
Objective: Our aim was to develop a rapid, economical, and environmentally safe method for extraction of high-quality genomic DNA, which can be used to determine clinically important genotypes from trace quantity samples and which has sufficient yield for multiple assays.
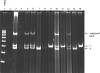
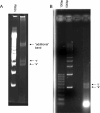
Methods: We developed a method of extracting high-quality genomic DNA from buccal swab, which we termed the 'rapid method for swab' (RMS). We compared RMS with two established procedures, specifically the original rapid method and the commercially available Buccal Amp method. We assessed the generated genomic DNAs by their (i) quality, (ii) quantity, (iii) restriction enzyme digestibility, and (iv) PCR-based genotyping in addition to time, cost, and environmental impact of the procedures.
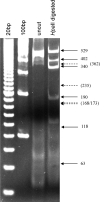
Main results: DNA generated by RMS was of higher purity than that by Buccal Amp. RMS is nonenzymatic and does not use strong chaotropic salts or extreme pH. We also showed the suitability of RMS-DNA for LA/LG genotyping as generated by PCR using 7-deaza-dGTP.
Conclusion: The RMS procedure is novel, efficient, safe, and yields sufficient material for multiple genotyping analyses. The RMS produces DNA of high quality from a single human buccal swab. RMS is a noninvasive technique and particularly suitable for children and older individuals and in field collection settings.
Figures




References
-
- Becker K, El-Faddagh M, Schmidt MH, Laucht M. Is the serotonin transporter polymorphism (5-HTTLPR) associated with harm avoidance and internalising problems in childhood and adolescence? J Neural Transm. 2007;114:395–402. - PubMed
-
- Brocke B, Armbruster D, Muller J, Hensch T, Jacob CP, Lesch KP, et al. Serotonin transporter gene variation impacts innate fear processing: acoustic startle response and emotional startle. Mol Psychiatry. 2006;11:1106–1112. - PubMed
-
- Bullido MJ, Artiga MJ, Recuero M, Sastre I, Garcia MA, Aldudo J, et al. A polymorphism in the regulatory region of APOE associated with risk for Alzheimer's dementia. Nat Genet. 1998;18:69–71. - PubMed
-
- Cervilla JA, Rivera M, Molina E, Torres-González F, Bellón JA, Moreno B, et al. The 5-HTTLPR s/s genotype at the serotonin transporter gene (SLC6A4) increases the risk for depression in a large cohort of primary care attendees: The PREDICT-gene study. Am J Med Genet B Neuropsych Genet. 2006;141B:912–917. - PubMed
-
- Covault J, Tennen H, Armeli S, Conner TS, Herman AI, Cillessen AH, et al. Interactive effects of the serotonin transporter 5-HTTLPR polymorphism and stressful life events on college student drinking and drug use. Biol Psychiatry. 2007;61:609–616. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

