Suppression of induced pluripotent stem cell generation by the p53-p21 pathway
- PMID: 19668191
- PMCID: PMC2917235
- DOI: 10.1038/nature08235
Suppression of induced pluripotent stem cell generation by the p53-p21 pathway
Abstract
Induced pluripotent stem (iPS) cells can be generated from somatic cells by the introduction of Oct3/4 (also known as Pou5f1), Sox2, Klf4 and c-Myc, in mouse and in human. The efficiency of this process, however, is low. Pluripotency can be induced without c-Myc, but with even lower efficiency. A p53 (also known as TP53 in humans and Trp53 in mice) short-interfering RNA (siRNA) was recently shown to promote human iPS cell generation, but the specificity and mechanisms remain to be determined. Here we report that up to 10% of transduced mouse embryonic fibroblasts lacking p53 became iPS cells, even without the Myc retrovirus. The p53 deletion also promoted the induction of integration-free mouse iPS cells with plasmid transfection. Furthermore, in the p53-null background, iPS cells were generated from terminally differentiated T lymphocytes. The suppression of p53 also increased the efficiency of human iPS cell generation. DNA microarray analyses identified 34 p53-regulated genes that are common in mouse and human fibroblasts. Functional analyses of these genes demonstrate that the p53-p21 pathway serves as a barrier not only in tumorigenicity, but also in iPS cell generation.
Figures
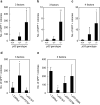
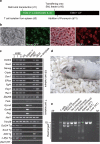
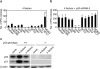

Comment in
-
Stem cells: The promises and perils of p53.Nature. 2009 Aug 27;460(7259):1085-6. doi: 10.1038/4601085a. Nature. 2009. PMID: 19713919 Free PMC article.
References
-
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. - PubMed
-
- Maherali N, et al. Directly reprogrammed fibroblasts show global epigenetic remodelling and widespread tissue contribution. Cell Stem Cell. 2007;1(1):55–70. - PubMed
-
- Okita K, Ichisaka T, Yamanaka S. Generation of germ-line competent induced pluripotent stem cells. Nature. 2007;448:313–317. - PubMed
-
- Wernig M, et al. In vitro reprogramming of fibroblasts into a pluripotent ES cell-like state. Nature. 2007;448:318–324. - PubMed
-
- Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

