The inadequacy of morphology for species and genus delineation in microbial eukaryotes: an example from the parabasalian termite symbiont coronympha
- PMID: 19668363
- PMCID: PMC2719052
- DOI: 10.1371/journal.pone.0006577
The inadequacy of morphology for species and genus delineation in microbial eukaryotes: an example from the parabasalian termite symbiont coronympha
Abstract
Background: For the majority of microbial eukaryotes (protists, algae), there is no clearly superior species concept that is consistently applied. In the absence of a practical biological species concept, most species and genus level delineations have historically been based on morphology, which may lead to an underestimate of the diversity of microbial eukaryotes. Indeed, a growing body of molecular evidence, such as barcoding surveys, is beginning to support the conclusion that significant cryptic species diversity exists. This underestimate of diversity appears to be due to a combination of using morphology as the sole basis for assessing diversity and our inability to culture the vast majority of microbial life. Here we have used molecular markers to assess the species delineations in two related but morphologically distinct genera of uncultivated symbionts found in the hindgut of termites.
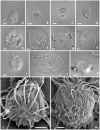
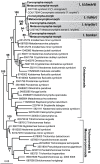
Methodology/principal findings: Using single-cell isolation and environmental PCR, we have used a barcoding approach to characterize the diversity of Coronympha and Metacoronympha symbionts in four species of Incisitermes termites, which were also examined using scanning electron microscopy and light microcopy. Despite the fact that these genera are significantly different in morphological complexity and structural organisation, we find they are two life history stages of the same species. At the same time, we show that the symbionts from different termite hosts show an equal or greater level of sequence diversity than do the hosts, despite the fact that the symbionts are all classified as one species.
Conclusions/significance: The morphological information used to describe the diversity of these microbial symbionts is misleading at both the genus and species levels, and led to an underestimate of species level diversity as well as an overestimate of genus level diversity. The genus 'Metacoronympha' is invalid and appears to be a life history stage of Coronympha, while the single recognized species of Coronympha octonaria inhabiting these four termites is better described as four distinct species.
Conflict of interest statement
Figures

References
-
- Gibson W. Resolution of the species problem in African trypanosomes. Int J Parasitol. 2007;37:829–838. - PubMed
-
- Heitman J. Sexual reproduction and the evolution of microbial pathogens. Curr Biol. 2006;16:R711–725. - PubMed
-
- Gibson W, Garside L. Genetic exchange in Trypanosoma brucei brucei: variable chromosomal location of housekeeping genes in different trypanosome stocks. Mol Biochem Parasitol. 1991;45:77–89. - PubMed
-
- Bell G. Cambridge: Cambridge University Press; 1988. Sex and death in protozoa.199
Publication types
MeSH terms
Associated data
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

