Phosphorylation of Nrf2 at multiple sites by MAP kinases has a limited contribution in modulating the Nrf2-dependent antioxidant response
- PMID: 19668370
- PMCID: PMC2719090
- DOI: 10.1371/journal.pone.0006588
Phosphorylation of Nrf2 at multiple sites by MAP kinases has a limited contribution in modulating the Nrf2-dependent antioxidant response
Abstract
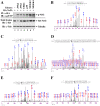
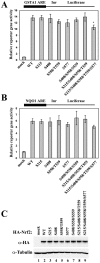
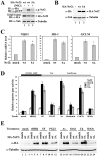
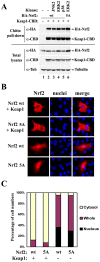
The bZIP transcription factor Nrf2 has emerged as a pivotal regulator of intracellular redox homeostasis by controlling the expression of many endogenous antioxidants and phase II detoxification enzymes. Upon oxidative stress, Nrf2 is induced at protein levels through redox-sensitive modifications on cysteine residues of Keap1, a component of the E3 ubiquitin ligase that targets Nrf2 for ubiquitin-dependent degradation. The mitogen activated protein kinases (MAPKs) have previously been proposed to regulate Nrf2 in response to oxidative stress. However, the exact role of MAPKs and the underlying molecular mechanism remain poorly defined. Here we report the first evidence that Nrf2 is phosphorylated in vivo by MAPKs. We have identified multiple serine/threonine residues as major targets of MAPK-mediated phosphorylation. Combined alanine substitution on those residues leads to a moderate decrease in the transcriptional activity of Nrf2, most likely due to a slight reduction in its nuclear accumulation. More importantly, Nrf2 protein stability, primarily controlled by Keap1, is not altered by Nrf2 phosphorylation in vivo. These data indicate that direct phosphorylation of Nrf2 by MAPKs has limited contribution in modulating Nrf2 activity. We suggest that MAPKs regulate the Nrf2 signaling pathway mainly through indirect mechanisms.
Conflict of interest statement
Figures
Similar articles
-
Review of molecular mechanisms involved in the activation of the Nrf2-ARE signaling pathway by chemopreventive agents.Methods Mol Biol. 2010;647:37-74. doi: 10.1007/978-1-60761-738-9_3. Methods Mol Biol. 2010. PMID: 20694660 Review.
-
Acetylation of Nrf2 by p300/CBP augments promoter-specific DNA binding of Nrf2 during the antioxidant response.Mol Cell Biol. 2009 May;29(10):2658-72. doi: 10.1128/MCB.01639-08. Epub 2009 Mar 9. Mol Cell Biol. 2009. PMID: 19273602 Free PMC article.
-
Keap1 controls postinduction repression of the Nrf2-mediated antioxidant response by escorting nuclear export of Nrf2.Mol Cell Biol. 2007 Sep;27(18):6334-49. doi: 10.1128/MCB.00630-07. Epub 2007 Jul 16. Mol Cell Biol. 2007. PMID: 17636022 Free PMC article.
-
Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex.Mol Cell Biol. 2004 Dec;24(24):10941-53. doi: 10.1128/MCB.24.24.10941-10953.2004. Mol Cell Biol. 2004. PMID: 15572695 Free PMC article.
-
Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals.Planta Med. 2008 Oct;74(13):1526-39. doi: 10.1055/s-0028-1088302. Epub 2008 Oct 20. Planta Med. 2008. PMID: 18937164 Review.
Cited by
-
Cysteine Sulfenylation Directs IRE-1 to Activate the SKN-1/Nrf2 Antioxidant Response.Mol Cell. 2016 Aug 18;63(4):553-566. doi: 10.1016/j.molcel.2016.07.019. Mol Cell. 2016. PMID: 27540856 Free PMC article.
-
Identification and functional studies of a new Nrf2 partner IQGAP1: a critical role in the stability and transactivation of Nrf2.Antioxid Redox Signal. 2013 Jul 10;19(2):89-101. doi: 10.1089/ars.2012.4586. Epub 2012 Sep 17. Antioxid Redox Signal. 2013. PMID: 22793650 Free PMC article.
-
Biochemical basis of the antidiabetic activity of oleanolic acid and related pentacyclic triterpenes.Diabetes. 2013 Jun;62(6):1791-9. doi: 10.2337/db12-1215. Diabetes. 2013. PMID: 23704520 Free PMC article. Review.
-
Arginine methylation of SKN-1 promotes oxidative stress resistance in Caenorhabditis elegans.Redox Biol. 2019 Feb;21:101111. doi: 10.1016/j.redox.2019.101111. Epub 2019 Jan 15. Redox Biol. 2019. PMID: 30682707 Free PMC article.
-
Pro-Inflammatory Serum Amyloid a Stimulates Renal Dysfunction and Enhances Atherosclerosis in Apo E-Deficient Mice.Int J Mol Sci. 2021 Nov 22;22(22):12582. doi: 10.3390/ijms222212582. Int J Mol Sci. 2021. PMID: 34830462 Free PMC article.
References
-
- Yang CS, Landau JM, Huang MT, Newmark HL. Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu Rev Nutr. 2001;21:381–406. - PubMed
-
- Kanwar JR. Anti-inflammatory immunotherapy for multiple sclerosis/experimental autoimmune encephalomyelitis (EAE) disease. Curr Med Chem. 2005;12:2947–2962. - PubMed
-
- Klaunig JE, Kamendulis LM. The role of oxidative stress in carcinogenesis. Annu Rev Pharmacol Toxicol. 2004;44:239–267. - PubMed
-
- Ruef J, Peter K, Nordt TK, Runge MS, Kubler W, et al. Oxidative stress and atherosclerosis: its relationship to growth factors, thrombus formation and therapeutic approaches. Thromb Haemost. 1999;82(Suppl 1):32–37. - PubMed
-
- Simonian NA, Coyle JT. Oxidative stress in neurodegenerative diseases. Annu Rev Pharmacol Toxicol. 1996;36:83–106. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

