Treatment of age-related macular degeneration: focus on ranibizumab
- PMID: 19668384
- PMCID: PMC2698673
- DOI: 10.2147/opth.s1959
Treatment of age-related macular degeneration: focus on ranibizumab
Abstract
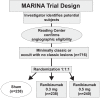
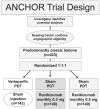
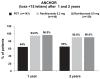
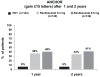
Ranibizumab, a humanized antigen-binding fragment (Fab) that binds all isoforms of VEGF-A, significantly slows down loss of vision and causes significant visual improvement in many patients with choroidal neovascularization (CNV) due to exudative age-related macular degeneration (AMD). These benefits of intravitreal ranibizumab apply to all angiographic subtypes of neovascular AMD and across all lesion sizes when the drug is injected at monthly intervals as shown in two pivotal phase III trials (ANCHOR and MARINA). The results from the PrONTO study suggest that less frequent treatment with ranibizumab through a variable dosing regimen dependent on optical coherence tomography (OCT) findings is a treatment option that results in comparably favorable visual outcomes. Currently, it is unclear whether combination therapy of ranibizumab with photodynamic therapy (PDT) provides any significant advantage over ranibizumab monotherapy (FOCUS trial); however, the combination of PDT and ranibizumab may decrease the need for frequent retreatment. This question will be addressed in the SUMMIT trial. Therapy with ranibizumab is generally very well tolerated with a low rate of seriously adverse ocular events or systemic side-effects. The advent of vascular endothelial growth factor (VEGF) inhibitors has revolutionized the therapy of neovascular AMD. Ranibizumab at the moment appears to be the most effective approved treatment for neovascular AMD.
Keywords: Lucentis; age-related macular degeneration (AMD); exudative AMD; neovascular; ranibizumab; treatment; vascular endothelial growth factor (VEGF).
Figures

References
-
- Adamis AP, Miller JW, Bernal MT, et al. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol. 1994;118:445–50. - PubMed
-
- Adamis AP, Shima DT, Tolentino MJ, et al. Inhibition of vascular endothelial growth factor prevents retinal ischemia-associated iris neovascularization in a nonhuman primate. Arch Ophthalmol. 1996;114:66–71. - PubMed
-
- Adams GP, Weiner LM. Monoclonal antibody therapy of cancer. Nat Biotech. 2005;23:1147–57. - PubMed
-
- Aisenbrey S, Lafaut BA, Szurman P, et al. Macular translocation with 360 degrees retinotomy for exudative age-related macular degeneration. Arch Ophthalmol. 2002;120:451–9. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

