Pooled results of two randomized clinical trials comparing the efficacy and safety of travoprost 0.004%/timolol 0.5% in fixed combination versus concomitant travoprost 0.004% and timolol 0.5%
- PMID: 19668487
- PMCID: PMC2701118
Pooled results of two randomized clinical trials comparing the efficacy and safety of travoprost 0.004%/timolol 0.5% in fixed combination versus concomitant travoprost 0.004% and timolol 0.5%
Abstract
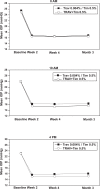
Purpose: To compare the IOP-lowering efficacy of the fixed combination of travoprost 0.004%/timolol 0.5% dosed once daily in the morning with the concomitant administration of travoprost 0.004% dosed once daily in the evening and timolol 0.5% dosed once daily in the morning.
Methods: This was an analysis of pooled data from two similarly designed prospective, randomized, controlled clinical trials comparing the fixed combination and concomitant therapy.
Results: Mean IOP ranged from 15.7 to 16.8 mmHg for the fixed combination group, and from 15.1 to 16.4 mmHg for the concomitant group. Mean IOP reductions were up to 9.0 mmHg in the fixed combination group, and up to 8.8 mmHg in the concomitant group. The differences in mean IOP change between treatment groups ranged from -0.2 to +0.9 mmHg across visits and time points. The safety profile was generally similar between groups. An exception was the incidence of ocular hyperemia, which was 13.7% with the fixed combination and 20.8% with concomitant therapy (p = 0.02).
Conclusion: The fixed combination of travoprost 0.004% and timolol 0.5% provides IOP-lowering efficacy that is similar to concomitant administration of travoprost 0.004% dosed once daily in the evening and timolol 0.5% dosed once daily in the morning.
Keywords: glaucoma; ocular hypertension; prostaglandin; timolol; travoprost.
Figures
References
-
- Alm A, Stjernschantz J. Effects on intraocular pressure and side effects of 0.005% latanoprost applied once daily, evening or morning. A comparison with timolol. Scandinavian latanoprost study group. Ophthalmology. 1995;102:1743–52. - PubMed
-
- Baun O, Heegaard S, Kessing SV, et al. The morphology of conjunctiva after long-term topical anti-glaucoma treatment. A quantitative analysis. Acta Ophthalmol Scand. 1995;73:242–5. - PubMed
-
- Broadway DC, Grierson I, O’Brien C, et al. Adverse effects of topical antiglaucoma medication. I. The conjunctival cell profile. Arch Ophthalmol. 1994a;112:1437–45. - PubMed
-
- Broadway DC, Grierson I, O’Brien C, et al. Adverse effects of topical antiglaucoma medication. II. The outcome of filtration surgery. Arch Ophthalmol. 1994b;112:1446–54. - PubMed
-
- Fechtner RD, Realini T. Fixed combinations of topical glaucoma medications. Curr Opin Ophthalmol. 2004;15:132–5. - PubMed
LinkOut - more resources
Full Text Sources