Intravitreal bevacizumab treatment for retinal neovascularization and vitreous hemorrhage in proliferative diabetic retinopathy
- PMID: 19668504
- PMCID: PMC2704517
Intravitreal bevacizumab treatment for retinal neovascularization and vitreous hemorrhage in proliferative diabetic retinopathy
Abstract
Purpose: To report the short term anatomical and visual acuity response after intravitreal injection of bevacizumab in patients with proliferative diabetic retinopathy.
Methods: Ten eyes having proliferative diabetic retinopathy were treated with at least one intravitreal injection of bevacizumab, 1.25 mg in 0.05 mL. Patients underwent complete ophthalmologic evaluation at baseline and follow up visits including Snellen's best corrected visual acuity (BCVA), fundus biomicroscopy and fluorescein angiography. Vitreous hemorrhage precluding laser treatment was present in 6 eyes.
Results: Follow up ranged between 3 and 6 months (mean 4.2 +/- 1.23 months). Improvement of vision was observed after one week with BCVA improved to 0.47 +/- 0.26 compared with baseline BCVA of 0.365 +/- 0.26 (P < 0.05). At last follow up, BCVA was 0.65 +/- 0.33. Seven eyes had better visual acuity. Vitreous hemorrhage cleared completely in 4 eyes while 2 eyes had residual vitreous hemorrhage. Regression of retinal neovascularization was complete in 7 eyes, incomplete in 3 eyes. Regression was observed as early as 2 weeks after first injection. Reperfusion after regression was observed in 2 eyes. Patients received a mean of 1.7 +/- 0.67 injections per eye. No complications were noted in all eyes. Pancryopexy was done in 1 eye and additional laser was done in 2 eyes.
Conclusion: Intravitreal bevacizumab was safe and effective in treatment of proliferative diabetic retinopathy. It can induce effective regression of retinal neovascularization and rapid clearance of vitreous hemorrhage. It can be used as an adjunctive therapy with laser photocoagulation and to enhance absorption of vitreous hemorrhage with subsequent deferral from vitrectomy.
Summary: Ten eyes having proliferative diabetic retinopathy were treated with intravitreal bevacizumab. Following injection, evident regression of retinal neovascularization and rapid clearance of vitreous hemorrhage as well as vision improvement was observed.
Keywords: intravitreal bevacizumab; proliferative diabetic retinopathy; retinal neovascularization; vascular endothelial growth factor; vitreous hemorrhage.
Figures

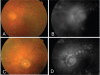
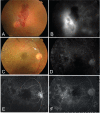
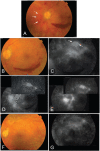
References
-
- Aiello LP, Brucker AJ, Chang S, et al. Evolving guidelines for intravitreal injections. Retina. 2004;24:13–19. - PubMed
-
- Arevalo JF, Wu L, Sanchez JG, et al. Intravitreal bevacizumab (Avastin) for proliferative diabetic retinopathy. Program and abstracts of “Cannes Retina Festival”; the joint meeting of the American Society of Retina Specialists and European Vitreoretinal Society; September 9–13; 2006. p. 213.
-
- Avery RL. Regression of retina and iris neovascularization after Intravitreal bevacizumab (Avastin) treatment. Retina. 2006;26:352–3. - PubMed
-
- Avery RL, Pieramici DJ, Castellarine AA, et al. Intravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Program and abstracts of “Cannes Retina Festival”; the joint meeting of the American Society of Retina Specialists and European Vitreoretinal Society; September 9–13; 2006. p. 139.
-
- Bakri SJ, Snyder M, Pulido JS, et al. Pharmacokinetics of Intravitreal bevacizumab (Avastin). Program and abstracts of “Cannes Retina Festival”; the joint meeting of the American Society of Retina Specialists and European Vitreoretinal Society; September 9–13; 2006. p. 77.
LinkOut - more resources
Full Text Sources
Medical

