Ganciclovir ophthalmic gel, 0.15%: a valuable tool for treating ocular herpes
- PMID: 19668521
- PMCID: PMC2704535
Ganciclovir ophthalmic gel, 0.15%: a valuable tool for treating ocular herpes
Abstract
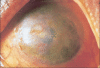
Ocular herpes simplex virus (HSV) infection remains a major cause of corneal blindness. Several topical and oral antiviral medications have been used to treat herpetic keratitis. Advances in topical ophthalmic antivirals have been made over the past several decades. The first antivirals that were discovered were cytotoxic, while the antivirals developed more recently, such as acyclovir and ganciclovir, have exceeded these drugs in both efficacy and tolerability. Commercially available outside of the US since 1996, ganciclovir ophthalmic gel, 0.15% (GCV 0.15%, European tradename: Virgan((R))) is sold in more than 30 countries and has become the standard of care in treating acute herpetic keratitis. GCV 0.15% has been studied in animal models of ocular herpes, in healthy volunteers, and in several clinical studies. It has been found to be safe and effective at treating acute superficial herpetic keratitis. Previous preclinical studies of ganciclovir have shown activity against several common adenovirus strains and one recent clinical study demonstrated clinical effect against adenoviral conjunctivitis. This review is intended to provide a comprehensive overview of the GCV 0.15%, including a brief summary of the etiology and available treatments for ocular HSV, an explanation of GCV 0.15% mechanism of action, a compendium of preclinical and clinical GCV 0.15% studies, and an introduction into new areas of interest involving this drug.
Keywords: adenovirus; antiviral; ganciclovir; herpes simplex virus; herpetic keratitis.
Figures



References
-
- Biser SA, Perry HD.Corneal and anterior segment diseases – herpes simplex keratitis Ophthalmic Hyperguide: Corneal and Anterior Segment Diseases c2006[cited 2007 Jan 10] URL: http://www.ophthalmic.hyperguides.com/default.asp?section=body.asp
-
- Bio-Tox . Vallauris, France: Rapport No. BT2360; 1990. Determination de l'agressivite oculaire du gel ophtalmique GV 550 par applications iteratives topiques pendant six semaines chez le lapin.
-
- Bruno B, Gooley T, Hackman RC, et al. Adenovirus infection in hematopoietic stem cell transplantation: effect of ganciclovir and impact on survival. Biol Blood Marrow Transplant. 2003;9:341–52. - PubMed
-
- Castela N, Vermerie N, Chast F, et al. Ganciclovir ophthalmic gel in herpes simplex virus rabbit keratitis: intraocular penetration and efficacy. J Ocul Pharmacol. 1994;10:439–51. - PubMed
-
- Clirophta . Evaluation de la tolérance locale chez le volontaire sain, après instillations multiples d'un gel ophtalmique de ganciclovir 0,15%, Virgan. Nice, France: Rapport clinique Virgan/F-94-01. Dossier d'AMM; 1994a.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

