The role of difluprednate ophthalmic emulsion in clinical practice
- PMID: 19668594
- PMCID: PMC2709030
- DOI: 10.2147/opth.s4460
The role of difluprednate ophthalmic emulsion in clinical practice
Abstract
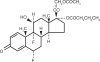
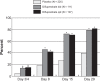
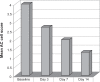
The mainstay in the treatment of ocular inflammation, either post-surgical or endogenous, is the use of steroids. While these agents effectively address inflammation, they are not without their risks, including ocular hypertension and acceleration of cataract formation. The most notorious culprits are the strong steroids, such as prednisolone acetate and betamethasone. This review aims to cover the biochemistry and drug development of difluprednate, a novel synthetic strong steroid emulsion. In vivo pharmacokinetics as well as ocular distribution and metabolism are discussed, followed by a comprehensive summary of phase I, II, and III clinical trials evaluating safety and efficacy in patients suffering from postoperative inflammation or anterior uveitis. The objective is to provide an increased familiarity with this newly approved medication as a welcome addition to the ophthalmologist's armamentarium.
Keywords: difluprednate; emulsion; ophthalmic surgery; steroid.
Figures




References
-
- Apple DJ, Solomon KD, Tetz MR, et al. Posterior capsule opacification. Surv Ophthalmol. 1992;37:73–116. - PubMed
-
- Miyake K, Maekubo K, Miyake Y, et al. Pupillary fibrin membrane; a frequent early complication after posterior chamber lens implantation in Japan. Ophthalmol. 1989;96:1228–1233. - PubMed
-
- Schimmer BP, Parker KL. Adrenocorticotropic hormone; adrenocortical steroids and their synthetic analogs; inhibitors of the synthesis and actions of adrenocortical hormones. In: Hardman JG, Limbird LE, Gilman AG, editors. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 10th Edition. New York: McGraw–Hill; 2001. pp. 1649–1677.
-
- Sanders DR, Kraff M. Steroidal and nonsteroidal anti-inflammatory agents. Effect on postsurgical inflammation and blood–aqueous humor barrier breakdown. Arch Ophthalmol. 1984;102(10):1453–1456. - PubMed
-
- Korenfeld M. Difluprednate: changing the landscape of ocular pharmacology. Expert Rev Ophthamol. 2008;3(6):619–625.
LinkOut - more resources
Full Text Sources
Other Literature Sources

