A novel gammaD-crystallin mutation causes mild changes in protein properties but leads to congenital coralliform cataract
- PMID: 19668596
- PMCID: PMC2722711
A novel gammaD-crystallin mutation causes mild changes in protein properties but leads to congenital coralliform cataract
Abstract
Purpose: To identify the genetic lesions for congenital coralliform cataract.
Methods: Two Chinese families with autosomal dominant coralliform cataract, 12 affected and 14 unaffected individuals, were recruited. Fifteen known genes associated with autosomal dominant congenital cataract were screened by two-point linkage analysis with gene based single nucleotide polymorphisms and microsatellite markers. Sequence variations were identified. Recombinant FLAG-tagged wild type or mutant gammaD-crystallin was expressed in human lens epithelial cells and COS-7 cells. Protein solubility and intracellular distribution were analyzed by western blotting and immunofluorescence, respectively.
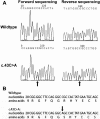
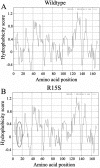
Results: A novel heterozygous change, c.43C>A (R15S) of gammaD-crystallin (CRYGD) co-segregated with coralliform cataract in one family and a known substitution, c.70C>A (P24T), in the other family. Unaffected family members and 103 unrelated control subjects did not carry these mutations. Similar to the wild type protein, R15S gammaD-crystallin was detergent soluble and was located in the cytoplasm. ProtScale and ScanProsite analyses revealed raised local hydrophobicity and the creation of a hypothetical casein kinase II phosphorylation site.
Conclusions: A novel R15S mutation caused congenital coralliform cataract in a Chinese family. R15S possessed similar properties to the wild type gammaD-crystallin, but its predicted increase of hydrophobicity and putative phosphorylation site could lead to protein aggregation, subsequently causing opacification in lens.
Figures


References
-
- Abrahamsson M, Magnusson G, Sjostrom A, Popovic Z, Sjostrand J. The occurrence of congenital cataract in western Sweden. Acta Ophthalmol Scand. 1999;77:578–80. - PubMed
-
- Rahi JS, Dezateux C. Measuring and interpreting the incidence of congenital ocular anomalies: lessons from a national study of congenital cataract in the UK. Invest Ophthalmol Vis Sci. 2001;42:1444–8. - PubMed
-
- Holmes JM, Leske DA, Burke JP, Hodge DO. Birth prevalence of visually significant infantile cataract in a defined U.S. population. Ophthalmic Epidemiol. 2003;10:67–74. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
