Merkel cells as putative regulatory cells in skin disorders: an in vitro study
- PMID: 19668696
- PMCID: PMC2722079
- DOI: 10.1371/journal.pone.0006528
Merkel cells as putative regulatory cells in skin disorders: an in vitro study
Abstract
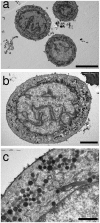
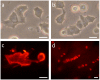
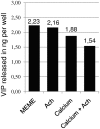
Merkel cells (MCs) are involved in mechanoreception, but several lines of evidence suggest that they may also participate in skin disorders through the release of neuropeptides and hormones. In addition, MC hyperplasias have been reported in inflammatory skin diseases. However, neither proliferation nor reactions to the epidermal environment have been demonstrated. We established a culture model enriched in swine MCs to analyze their proliferative capability and to discover MC survival factors and modulators of MC neuroendocrine properties. In culture, MCs reacted to bFGF by extending outgrowths. Conversely, neurotrophins failed to induce cell spreading, suggesting that they do not act as a growth factor for MCs. For the first time, we provide evidence of proliferation in culture through Ki-67 immunoreactivity. We also found that MCs reacted to histamine or activation of the proton gated/osmoreceptor TRPV4 by releasing vasoactive intestinal peptide (VIP). Since VIP is involved in many pathophysiological processes, its release suggests a putative regulatory role for MCs in skin disorders. Moreover, in contrast to mechanotransduction, neuropeptide exocytosis was Ca(2+)-independent, as inhibition of Ca(2+) channels or culture in the absence of Ca(2+) failed to decrease the amount of VIP released. We conclude that neuropeptide release and neurotransmitter exocytosis may be two distinct pathways that are differentially regulated.
Conflict of interest statement
Figures





References
-
- Moll I, Roessler M, Brandner JM, Eispert AC, Houdek P, et al. Human Merkel cells–aspects of cell biology, distribution and functions. Eur J Cell Biol. 2005;84:259–271. - PubMed
-
- Halata Z, Grim M, Bauman KI. Friedrich Sigmund Merkel and his “Merkel cell”, morphology, development, and physiology: review and new results. Anat Rec A Discov Mol Cell Evol Biol. 2003;271:225–239. - PubMed
-
- Boulais N, Misery L. Merkel cells. J Am Acad Dermatol. 2007;57:147–165. - PubMed
-
- Ogawa H. The Merkel cell as a possible mechanoreceptor cell. Prog Neurobiol. 1996;49:317–334. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

