Topical ophthalmic NSAIDs: a discussion with focus on nepafenac ophthalmic suspension
- PMID: 19668727
- PMCID: PMC2693998
- DOI: 10.2147/opth.s1067
Topical ophthalmic NSAIDs: a discussion with focus on nepafenac ophthalmic suspension
Abstract
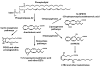
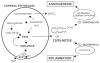
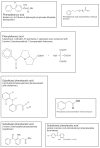
The removal of diclofenac sodium ophthalmic solution as a viable pharmaceutical entity in September 1999 from the US market spurred considerable interest in the general safety and effectiveness of topical ophthalmic NSAIDs for treatment of anterior segment inflammation. In late 1999 the use of topical ocular NSAIDs declined in the US as a result of incidents involving corneal melts and toxicity surrounding use of generic diclofenac. However, since the removal of diclofenac sodium ophthalmic solution from the marketplace, ophthalmic NSAIDs have regained use as viable pharmacotherapeutic entities. Moreover, several new ophthalmic NSAID products have recently been introduced for commercial use in the US including the novel chemical entity nepafenac. The purpose of this report is to revisit the use of topical ophthalmic NSAIDs for the treatment of surgically induced anterior segment inflammation with a particular focus on nepafenac. Nepafenac is unique among ophthalmic NSAIDs in that it is a prodrug deaminated to amfenac, a highly effective non-selective cyclooxygenase inhibitor. In the case of topical ophthalmic NSAIDs, practitioners should carefully weigh the cost-benefit of implementing "highly potent" new drug products because perturbations in pharmacodynamic response due to the inherent novelty in terms of chemical designs may outweigh the demonstrated replicative pharmacologic action of all topical ophthalmic NSAIDs.
Keywords: nepafenac; ocular inflammatory disease; ophthalmic NSAIDs.
Figures

References
-
- Acosta MC, Luna C, Graff G, et al. Comparative effects of the non-steroidal anti-inflammatory drug nepafenac on corneal sensory nerve fibers responding to chemical irritation. Investig Ophthalmol Vis Sci. 2007;48:182–8. - PubMed
-
- Acular® (ketorolac tromethamine) package labeling. Allergan Inc; Irvine, CA., USA: 2003.
-
- AIDS Info. US Department of Health and Human Services; [Accessed Jan 2008]. Carbomer 974. URL: http://aidsinfo.nih.gov/DrugsNew/DrugDetailNT.aspx?MenuItem+Drugs&Search....
-
- Asai T, Nakagami T, Mochizuki M, et al. Three cases of corneal melting after instillation of a new non-steroidal anti-inflammatory drug. Cornea. 2006;25:224–7. - PubMed
-
- Bhatia AJ, Wade GN. Effects of pregnancy and ovarian steroids on fatty acid synthesis and uptake in Syrian hamsters. Am J Physiol. 1991;260:R153–R8. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

