Hepatitis B reactivation after chemotherapy: two decades of clinical research
- PMID: 19669300
- PMCID: PMC2716860
- DOI: 10.1007/s12072-008-9056-3
Hepatitis B reactivation after chemotherapy: two decades of clinical research
Abstract
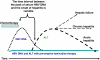
Hepatitis due to hepatitis B virus reactivation after cytotoxic or immunosuppressive therapy is a serious cause of liver-related morbidity and mortality. With the characterization of the underlying pathogenesis, much progress in the management of this important clinical problem has been made in the past 2 decades. By year 2008, it is mandatory to screen for hepatitis B surface antigen status before initiating intensive chemotherapy or immunosuppressive therapy. All those who are hepatitis B surface antigen positive should be started on preemptive nucleos(t)ide analogues. However, there remains important issues, such as the type and duration of nucleos(t)ide analogue therapy, which need to be understood. As not all hepatitis B surface antigen-positive patients will suffer from HBV reactivation, it is therefore useful to identify risk factors related to HBV reactivation so that patients will not be treated unnecessarily with nucleos(t)ide analogues. To date, a high baseline level of viral replication, as reflected by high serum HBV DNA level, positive serum hepatitis B e antigen, and a high intrahepatic covalently closed circular DNA level, is the most important predictor for HBV reactivation. Recently, there has been an increased awareness of reactivation of occult hepatitis B virus, especially in hepatitis B virus endemic area, such as the Asia-Pacific region. Careful epidemiological study will be needed to clarify the impact of occult hepatitis B infection in patients treated with cytotoxic or immunosuppressive therapy.
Figures


References
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '10458258', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/10458258/'}]}
- Liang R, Lau GKK, Kwong YL. Chemotherapy and bone marrow transplantation for cancer patient who are also chronic hepatitis B carriers: a review of the problem. J Clin Oncol. 1999;17:394–98. - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '11590667', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/11590667/'}]}
- Luo XR, Yan AW, Liang R, Lau GKK. Hepatitis B virus (HBV) reactivation after cytotoxic or immunosuppressive therapy-pathogenesis and management. Rev Med Virol. 2001;11(5):287–9. - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '10532191', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/10532191/'}]}
- Lau GKK, Lee CK, Liang R. Hepatitis B and bone marrow transplantation. Crit Rev Oncol Hematol. 1999;31:71–6. - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '16440366', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/16440366/'}]}
- Yeo W, Johnson PJ. Diagnosis, prevention and management of hepatitis B virus reactivation during anticancer therapy. Hepatology. 2006;43(2):209–20. - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '17338776', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/17338776/'}]}
- Lalazar G, Rund D, Shouval D. Screening, prevention and treatment of viral hepatitis B reactivation in patients with haematological malignancies. Br J Haematol. 2007;136(5):699–712. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

