Statistical considerations for assessment of bioanalytical incurred sample reproducibility
- PMID: 19669410
- PMCID: PMC2758127
- DOI: 10.1208/s12248-009-9134-z
Statistical considerations for assessment of bioanalytical incurred sample reproducibility
Abstract
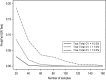
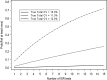
Bioanalytical method validation is generally conducted using standards and quality control (QC) samples which are prepared to be as similar as possible to the study samples (incurred samples) which are to be analyzed. However, there are a variety of circumstances in which the performance of a bioanalytical method when using standards and QCs may not adequately approximate that when using incurred samples. The objective of incurred sample reproducibility (ISR) testing is to demonstrate that a bioanalytical method will produce consistent results from study samples when re-analyzed on a separate occasion. The Third American Association of Pharmaceutical Scientists (AAPS)/Food and Drug Administration (FDA) Bioanalytical Workshop and subsequent workshops have led to widespread industry adoption of the so-called "4-6-20" rule for assessing incurred sample reproducibility (i.e. at least 66.7% of the re-analyzed incurred samples must agree within +/-20% of the original result), though the performance of this rule in the context of ISR testing has not yet been evaluated. This paper evaluates the performance of the 4-6-20 rule, provides general recommendations and guidance on appropriate experimental designs and sample sizes for ISR testing, discusses the impact of repeated ISR testing across multiple clinical studies, and proposes alternative acceptance criteria for ISR testing based on formal statistical methodology.
Figures






References
-
- Viswanathan CT, Bansal S, Booth B, DeStefano AJ, Rose MJ, Sailstad J, et al. Workshop/conference report - quantitative bioanalytical methods validation and implementation: best practices for chromatographic and ligand binding assays. AAPS J. 2007;9(1):E30–E42. doi: 10.1208/aapsj0901004. - DOI - PubMed
-
- Food and Drug Administration . Draft guidance for industry: bioanalytical method validation. Rockville, MD: US Food and Drug Administration; 1999.
MeSH terms
LinkOut - more resources
Full Text Sources

